1. Introduction
In Uruguay, as in other parts of the world, mastitis is the most common disease affecting cow welfare as well as the economic performance of dairy farms(1)(2). The mean prevalence of both clinical and subclinical mastitis in Uruguay is around 30%(2)(3), producing important economic losses(4). Prevention is the best way to reduce the prevalence of mastitis. Therefore, identifying risk factors that increase its incidence is crucial. Several cow- and herd-level risk factors for mastitis in Holstein cows have been identified; for instance, the cow´s parity, the housing type, and the herd size(2)(5)(6). However, other factors such as genetic strains, feeding strategies, and their interaction have not been studied sufficiently. It seems that different breeds(7) and their crossbreeding(8) would not influence udder health. However, Walsh and others(9) reported differences in somatic cell count (SCC) between several breeds, without an interaction between genotype and the environment.
North American (NA) Holstein Friesian is the most common dairy genetics (78%) in Uruguay(10). However, cows with New Zealand (NZ) origins (13%)(10) have increased due to some advantages such as smaller body size, higher milk fat and protein, superior fertility for seasonal calving, and enhanced foraging ability in pastoral systems. The advantages regarding udder health according to Holstein strains are not clear since some studies showed contradictory results. Lacy-Hulbert and others(11) reported higher SCC in NZ cows compared with NA cows at the end of lactation, but more cases of clinical mastitis in NA cows than in NZ cows at the beginning of lactation (cows under extended lactation). McClearn and others(8) did not find differences in SCC between the three genotypes of Holstein-Friesian. The incidence of clinical mastitis or differences in SCC by genotype could be due to udder conformation(12)(13), since NA cows would have a better-balanced udder and teats than NZ cows(14).
Furthermore, the most common feeding strategy in Uruguay is a mixed system, which is pasture based with silage and grain supplementation(10). The supplementation is offered on feedpads, most of which are concrete(10). The conditions of the feeding places (pasture and feedpads) are strongly influenced by climatic conditions (e.g., excessive mud during winter), and mastitis risk factors affecting cows may differ if the cows are maintained for a longer period on concrete or pasture. Pasture could be more beneficial for udder health as a result of less exposure to environmental pathogens, better teat hygiene, and/or lower stocking rate(13)(15)(16) than cows in confinement. However, grazing cows often have high mastitis incidence due to the bad conditions of the alleyways(17) or climatic stress, such as rainfall(18) and heat stress(19), which lead to exposure to pathogens and/or stress. Most of the information related to udder health in dairy cows has been generated in confinement systems, comparing different feeding strategies according to low or high concentrate. Studies comparing udder health in confinement systems and grazing systems consistently reported incongruous results. Sepúlveda Varas and others(20) reported a high incidence of both clinical and subclinical mastitis even in grazing herds. However, some epidemiological studies have found that the lack of access to pasture increased the risk of compromised udder health(21). To our knowledge, there are no publications about clinical mastitis frequency and SCC for NA and NZ Holstein Friesians under mixed- or pasture-based feeding strategies. It is possible to speculate that different genetics plus different feeding strategies could affect the incidence of udder diseases. Therefore, we hypothesized that dairy cows with NZ genetic origins might have a greater probability of clinical and subclinical mastitis than NA cows, and that animals under different feeding strategies will show different clinical and subclinical mastitis probability.
This study aimed to analyze the udder conformation, the probability of clinical mastitis, and the monthly SCC for NA and NZ Holstein-Friesian cows maintained in two different feeding strategies (pasture or mixed).
2. Materials and methods
2.1 Animals and husbandry conditions
An experiment in a farmlet study was carried out with 120 Holstein-Friesian cows over a one-lactation period (June 2019-May 2020), at the experimental station of the National Agricultural and Livestock Research Institute (INIA, by its Spanish acronym), Colonia, Uruguay (34◦20′ S, 57◦41′ W). A full explanation of the farmlet study is described in Stirling and others(22). Four groups were derived from the combination of two feeding strategies: Grass Maximum or Grass Fixed, and two Holstein strains: NZ or NA. The Grass Maximum and Grass Fixed feeding strategies were differentiated in the proportions of grazed pasture in the diet with the same level of concentrate per cow. The cows in Grass Fixed were fed an allowance of ⅓ pasture, ⅓ concentrate, and ⅓ silage. The silage was offered as a partial mixed ration on a concrete and dirt feedpad (4.5 m2/cow), and the total area (feeding and rest area) was 240 m2 per cow. The cows in Grass Maximum had a flexible pasture allowance determined by the pasture growth rate, which was estimated weekly, offering whole-crop silage on a feedpad as a buffer in case of pasture shortage. The pasture was a mix of Festuca arundinacea, Dactylis glomerata, Medicago sativa and Lolium multiflorum. The commercial concentrate was offered daily and individually in the milking parlor. Thirty NA and thirty NZ cows were randomly assigned to each treatment before calving, ensuring that groups were balanced for expected calving date, parity, milk yield, and milk fat content from the previous lactation. Also, all multiparous cows were selected for udder health [without clinical mastitis in previous lactation and low SCC (SCC ≤ 200.000) cells/ml]. The INIA dairy farm has a mastitis control protocol, which includes dry cow treatment with antibiotics and internal sealant for all cows(23). The cows were managed in a seasonal calving system (February-August), with most calving in autumn (April, May, June). All procedures were approved by the Ethical Committee of INIA (file number INIA.2019.11).
2.2 Clinical mastitis and somatic cell count
Clinical mastitis was defined as abnormal milk with the presence of clots, blood, pus, flakes, and/or changes in consistency or color accompanied or not by udder inflammation signs(24). Forestripping was used by the person in charge of milking to detect clinical mastitis at every milking. Cows diagnosed with clinical mastitis were treated immediately according to the treatment protocol recommended by the veterinarian and the milk was discharged. All clinical mastitis cases were registered in the dairy farm software, which included animal identification, date of diagnosis, treatment start date, affected teat(s), and drug(s) used for treatment.
The four groups of animals were milked two times per day (AM, PM) in a conventional milking parlor. Test-day milk samples were obtained every 15 days from two consecutive milkings (morning and afternoon milkings), and then SCC was analyzed using the mid-infrared method with Bentley 2000 equipment (Bentley Instruments Inc., Chaska, MN, USA). The samples collected during the first 3 days in milk were excluded from the evaluation due to colostrum.
2.3 Udder conformation
Udder conformation from all cows with an average (±SD) of 91.4 ± 36.1 days in milk was evaluated by a technician from the Uruguayan Society of Holstein Breeders (https://www.holando.com.uy), who observed each cow in a paddock from different body positions and assigned a point for each udder traits using a spreadsheet. The conformation traits evaluated were udder depth, udder texture, middle ligament, fore-udder attachment, front teat placement, rear-udder attachment length, rear-udder attachment width, rear teat placement, and teat length. The points were added up and each cow got a number (between 60 and 100 points) and a classification score [excellent (≥90), very good (85-89), double good (80-84), good (75-79), regular (70-74), bad (60-69)], which took into consideration the cow´s parity.
2.4 Statistical analysis
To analyze the relationship between each response variable (clinical mastitis presence/absence and SCC) and the explanatory variables (strains, feeding strategies, months in lactation, and parity), an exploratory data analysis was performed. The response variable SCC was transformed into the Somatic Cell Count Linear Score (SCCLS) according to the following transformation: Log2 (SCC/100) + 3 (National Cooperative Dairy Herd Improvement Program). The information obtained from the exploratory analysis was used to select the best family of models and the most relevant variables in the context of the hypotheses set up(25). Only cows that reached 10 months in lactation were considered.
To model the presence/absence of a clinical mastitis case as a function of the explanatory variables, a Binomial Generalized Linear Mixed Model (BGLMM) with a logit link function was used. The binomial distribution is typically used for binary outcomes. The logit function ensures the interpretation of the fitted values in terms of log odds and was presented as odds ratios (OR). Udder conformation categories for each strain were compared and tested through Fisher’s exact test, due to the lack of minimal expected observations required to use the Chi-square frequencies test.
The SCCLS as a response variable was subjected to a Linear Mixed Model (LMM) with Gaussian distribution and identity as the link function. In this case, the identity link is used for the canonical and Gaussian distribution as a consequence of the nature of the SCC transformation into SCCLS. Additionally, the residual structure was adjusted to include the auto-correlated structure of the longitudinal design.
In both models, strains (NZ or NA), time (months of lactation) feeding strategies (Grass Maximum or Grass Fixed) and parity (primiparous or multiparous) were fitted as fixed effects, and cows as random intercepts. Model goodness of fit was assessed by the calculation of marginal (R2m) and conditional (R2c) coefficient of determination for Generalized mixed-effect models. The α values for all statistical tests were assumed to be significant when <0.05. For both models, the variable selection was performed through Akaike Information Criterion (AIC), and the results are presented according to the optimal selected model.
For data analysis, the statistical software R(26) was used. Graphics were constructed using the “ggplot2” package; LMM models were fitted with the “nlme” package, and GLMM with “lme4” package. The R2 goodness of fit was estimated with the (r. squared GLMM) function implemented in the “MuMin” package.
3. Results
3.1 Clinical mastitis and somatic cell count
The probability of suffering clinical mastitis was significant for the interaction between parity and genetic strains (p=0.04) (Table 1), and for the month of lactation (p=0.02) (Table 1). For all groups, the odds of suffering clinical mastitis were low (OR<1). Nevertheless, differences between groups regarding the OR were found. The NA primiparous cows were the least likely to suffer clinical mastitis (OR: 0.003), while NA multiparous cows were the most likely to show clinical mastitis (OR: 0.12) (Figure 1). The NZ cows reported intermediate values of OR, where primiparous cows presented a value of 0.082, and multiparous of 0.066. For months in lactation, the odds of suffering clinical mastitis reduce by 0.84 as the month in lactation increases for both genetic strains (Figure 1).
The evolution over time of the SCCLS, considering parity, strains, and months in lactation, is presented in Figure 2. For the SCCLS, no significant differences were found for the explanatory variables considered.
Table 1
Binomial generalized linear mixed model (BGLMM) results of the probability
of suffering clinical mastitis by genetic and parity interaction across 10
months in lactation. The values are presented in the logit of odds scale
assuming North American strain and multiparous parity as references

CI: Confidence intervals for the estimated values.
R2 m: Marginal R2: represents the variance explained by the fixed effects.
R2c: Conditional R2: represents the variance explained by the entire model, including both fixed and random effects.
|
Fixed
Effects
|
|
Term
|
Estimate
|
P
value
|
CI
|
|
(Intercept)
|
-2.10
|
0.00
|
(-2.84:
-1.36)
|
|
Month
in lactation
|
-0.17
|
0.02
|
(-0.31:
-0.02)
|
|
Parity
= Primiparous
|
-1.49
|
0.01
|
(-2.62:
-0.36)
|
|
Genetic
= New Zealand
|
-0.61
|
0.08
|
(-1.30:
0.08)
|
|
Parity
= Primiparous × Genetic = New Zealand
|
1.65
|
0.04
|
(0.10:
3.20)
|
|
Random
Effects
|
|
Variable
|
Variance
(SD)
|
|
Individuals
|
3.14
(1.77)
|
|
Goodness
of fit
|
|
R2
m
|
9.1
%
|
|
R2
c
|
41.8
|
| | | | |
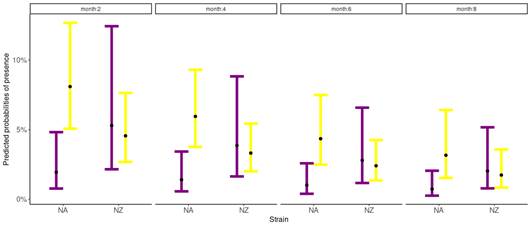 Figure
1
Predicted probabilities of clinical mastitis according to the Binomial
Generalized Linear Mixed Model, for both strains, North American (NA) and New
Zealand (NZ), for primiparous (purple) and multiparous (yellow)
Holstein-Friesian cows conditioned by the 2, 4, 6, and 8 months in lactation
Figure
1
Predicted probabilities of clinical mastitis according to the Binomial
Generalized Linear Mixed Model, for both strains, North American (NA) and New
Zealand (NZ), for primiparous (purple) and multiparous (yellow)
Holstein-Friesian cows conditioned by the 2, 4, 6, and 8 months in lactation
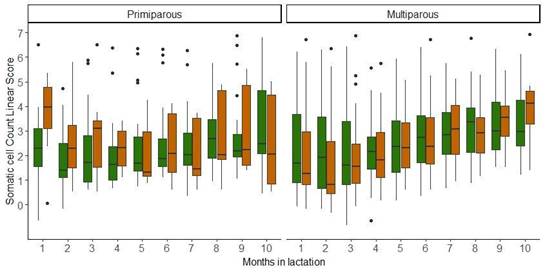 Figure 2
Descriptive statistics for the evolution of somatic cell count linear score
of two Holstein-Friesian strains (North American = green, New Zealand = brown)
by parity (primiparous and multiparous) and months in lactation
Figure 2
Descriptive statistics for the evolution of somatic cell count linear score
of two Holstein-Friesian strains (North American = green, New Zealand = brown)
by parity (primiparous and multiparous) and months in lactation
3.2 Body and udder conformation
There were no significant differences in udder conformation between NZ and NA cows (P=0.27) (Table 2).
Table 2
Percentage of cows with different udder conformation scores (excellent, very
good, double good, good, regular, bad) of two Holstein-Friesian strains (North
American: NA, and New Zealand: NZ)
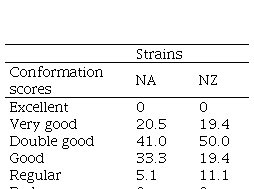
|
Strains
|
|
Conformation
scores
|
NA
|
NZ
|
|
Excellent
|
0
|
0
|
|
Very good
|
20.5
|
19.4
|
|
Double good
|
41.0
|
50.0
|
|
Good
|
33.3
|
19.4
|
|
Regular
|
5.1
|
11.1
|
|
Bad
|
0
|
0
|
4. Discussion
Clinical and subclinical mastitis is a multifactorial disease, with genetics being a possible cow-specific risk factor. Dairy breed differences on mastitis incidence have been reported previously(27), but information about the genetic origin within a breed is little and contradictory. Our study indicated a greater probability of clinical mastitis for multiparous Holstein NA cows, compared to other multiparous and primiparous groups, which could be partially explained by milk yield differences. In this farmlet study, the NA cows had a greater milk yield at the individual animal level than the NZ cows(22). Effectively, Lacy-Hulbert and others(11) reported more cases of clinical mastitis in multiparous NA cows (59%) compared with NZ cows (27%), and they associated it with greater milk yield in NA cows. On the other hand, there were fewer cases of clinical mastitis in primiparous cows of NA origin, which is online with the reported in Uruguay(14), and it has been associated with better udder conformation of NA cows(12)(28). However, in our study, there were no differences in udder conformation between Holstein strains; therefore, there would be other factors that might be playing an important role in our results, such as immunity, feeding or milk production level. Another possible explanation for the differences in clinical mastitis probability between the multiparous NA and primiparous (NA or NZ) might be given by the fact that mastitis increased with parity(29). A speculation is that there are differences in cisternal capacity, and contractility of the teat or teat canal length(30)(31) between Holstein strains, which are more evident when the number of calving increases. Unfortunately, immune response or udder anatomy were not evaluated in this study. Therefore, the clinical mastitis probability seems to depend on several factors that interact with strain (for example, parity) rather than only strain. Differences in the inherent mastitis resistance and/or efficient immune defense between the Holstein strain and parity should be investigated in the future.
Whole-lactation comparisons between pasture-based and confinement dairy systems under Uruguayan conditions are very limited. Internationally, a lower incidence of mastitis has been reported in pasture systems and attributed to a lower probability of exposure to environmental pathogens compared with confinement-housed cows(21)(27). The influence of the feeding system on clinical mastitis is related to cow cleanliness, which depends on climatic factors and alleyway conditions(32)(33)(34). Speculation is that in the present study there were no cleanliness differences in the place where feed was offered (pasture or feedpad), resulting in similar clinical mastitis probability. Unfortunately, this aspect was not assessed.
Despite Lacy-Hulbert and others(11) reporting greater SCC in NZ cows compared with NA cows, in a national study that compared primiparous NZ cows with NA no differences were found in SCC between the two Holsteins strains(14). Holstein cows in confinement had greater mean SCC than those in pasture-based systems(35)(36). However, as in our study, several studies failed to find a significant difference in SCC between confinement and pasture-based systems(27)(37)(38). Similar SCC between groups could have been due to the animals initially being selected for low SCC, and/or because the udder conformation was not different between strains. Therefore, the differences in udder health between Holstein strains were mainly observed in the clinical and not subclinical manifestations of the disease.
5. Conclusions
Regardless of the feeding strategy, the probability of clinical mastitis was affected by the strain of the Holstein cow (genetic origin of North America or New Zealand), but the differences were influenced by parity. The multiparous NA cows were the most susceptible to clinical mastitis; the opposite happened for primiparous NA cows. Feeding strategies or their interaction with the Holstein strain did not influence clinical mastitis or SCC. The SCC was not different between strains of Holstein cows.
Acknowledgments
The
authors thank the López family (milkers) for their help with the data
collection in the project, as well as Emilie Akkermans
for the help with language revision. Financial support was provided by the National
Agricultural and Livestock Research Institute [INIA, Route 50, 11 km, Colonia,
Uruguay (Grant No. N-18284 PL_21_0_00)].
References
1. Halasa T, Huijps K, Osteras O, Hogeveen H. Economic effects of bovine mastitis and mastitis management: a review. Vet Q. 2007;29:18-31.
2. Cruz I, Pereira I, Ruprechter G, Barca J, Meikle A, Larriestra A. Clinical disease incidence during early lactation, risk factors and association with fertility and culling in grazing dairy cows in Uruguay. Prev Vet Med. 2021;191:105-359.
3. de los Santos R, González-Revello A, Majul L, Umpiérrez A, Aldrovandi A, Gil A, Hirigoyen D, Zunino P. Subclinical bovine mastitis associated with Staphylococcus spp. in eleven Uruguayan dairy farms. J Infect Dev Ctries. 2022;6:630-7.
4. Gianneechini R, Parietti I, de María P. Evaluación de pérdidas económicas relacionadas a mastitis para establecimientos lecheros en Uruguay. In: Jornada de Lechería: 10 años de actividades del Laboratorio de Calidad de Leche. Montevideo: INIA; 2002. p. 35-9.
5. Bludau MJ, Maeschli A, Leiber F, Klocke P, Berezowski JA, Bodmer M, Vidondo B. The influence of the rearing period on intramammary infections in Swiss dairy heifers: a cross-sectional study. Prev Vet Med. 2016;129:23-34.
6. Vissio C, Pereyra ME, Lamberti MJ, Bonetto CC, Larriestra AJ. Mastitis en vacas Holstein y factores asociados en tambos de Córdoba. Rev Vet. 2020;31(2):120-5.
7. Lembeye F, Lopez-Villalobos N, Burke JL, Davis SR. Milk production of Holstein-Friesian, Jersey and crossbred cows milked once-a-day or twice-a-day in New Zealand. NZ J Agric Res. 2016;59:50-64.
8. McClearn B, Delaby L, Gilliland TJ, Guy C, Dineen M, Coughlan F, Buckley F, McCarthy B. An assessment of the production, reproduction and functional traits of Holstein-Friesian, Jersey × Holstein-Friesian and Norwegian Red × (Jersey × Holstein-Friesian) cows in pasture-based systems. J Dairy Sci. 2020;103(6):5200-14. doi: 10.3168/jds.2019-17476.
9. Walsh S, Buckley F, Berry DP, Rath M, Pierce K, Byrne N, Dillon P. Effects of breed, feeding system, and parity on udder health and milking characteristics. J Dairy Sci. 2007;90:5767-79.
10. Encuesta Lechera 2019: resultados preliminares [Internet]. Montevideo: INALE; 2019 [cited 2023 Jun 27]. Available from: https://www.inale.org/estadisticas/encuesta-lechera-2019-resultados-preliminares/
11. Lacy-Hulbert SJ, Summers EL, Williamson JH, Aspin PW, Kolver ES. Prevalence of mastitis for cows of different genotypes milked for two consecutive seasons. Proc N Z Soc Anim Prod. 2006;66:236-40.
12. Bludau MJ, Maeschli A, Leiber F, Steiner A, Klocke P. Mastitis in dairy heifers: prevalence and risk factors. Vet J. 2014;202:566-72.
13. Compton CW, Heuer C, Parker K, McDougall S. Risk factors for peripartum mastitis 17 in pasture-grazed dairy heifers. J Dairy Sci. 2007;90:4171-80.
14. Benítez J, Hernandorena J. Desempeño productivo, reproductivo y de salud de ubre de dos líneas genéticas de Holando uruguayo [grade’s thesis]. Montevideo (UY): Universidad de la República, Facultad de Veterinaria; 2015. 37p.
15. Parker KI, Compton C, Anniss FM, Weir A, Heuer C, McDougall S. Subclinical and clinical mastitis in heifers following the use of a teat sealant precalving. J Dairy Sci. 2007;90:207-18.
16. Schreiner DA, Ruegg PL. Relationship between udder and leg hygiene scores and subclinical mastitis. J Dairy Sci. 2003;86:3460-65.
17. Lopez-Benavides M, Williamson J, Pullinger G, Lacy-Hulbert S, Cursons R, Leigh J. Field observations on the variation of Streptococcus uberis populations in a pasture-based Dairy farm. J Dairy Sci. 2007;90(12):5558-66.
18. Sant’Anna AC, Paranhos da Costa MJ. The relationship between dairy cow hygiene and somatic cell count in milk. J Dairy Sci. 2011;94(8):3835-44.
19. Roman L, Saravia C, Astigarraga L, Bentancur O, La Manna A. Shade access in combination with sprinkling and ventilation effects performance of Holstein cows in early and late lactation. Anim Prod Sci. 2019;59:347-58.
20. Sepúlveda-Varas P, Weary DM, von Keyserlingk MA. Lying behavior and postpartum health status in grazing dairy cows. J Dairy Sci. 2014;97:6334-43.
21. Arnott G, Ferris CP, O’Connell NE. Welfare of dairy cows in continuously housed and pasture-based production systems. Animal. 2017;11(2):261-73.
22. Stirling S, Delaby L, Mendoza A, Fariña S. Intensification strategies for temperate hot-summer grazing dairy systems in South America: effects of feeding strategy and cow genotype. J Dairy Sci. 2021;104(12):12647-63.
23. de Torre E, Fariña S, Hirigoyen D, Lopez T, Martinez R, Morales T, Peña F, Pla M, Torres I, Viana H. Sistema de gestión para control y prevención de mastitis [Internet]. [place unknown]: GEA; 2016 [cited 2023 Jun 27]. 12p. Available from: https://bit.ly/3CVlC9v
24. Philpot NW, Nickerson S. Mastitis: el contra ataque. Ilionois: Surge International; 1992. 150p.
25. Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White JSS. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol. 2009;24(3):127-35.
26. Team RC. R: a language and environment for statistical computing [Internet]. Vienna: R Foundation for Statistical Computing; 2020 [cited 2023 Jun 27]. Avalaible from: https://www.R-project.org/
27. Washburn SP, White SL, Green Jr. JT, Benson GA. Reproduction, mastitis, and body condition of seasonally calved Holstein and Jersey cows in confinement or pasture systems. J Dairy Sci. 2002;85:105-11.
28. Steeneveld W, Hogeveen H, Barkema HW, van den Broek J, Huirne RBM. The influence of cow factors on the incidence of clinical mastitis in dairy cows. J Dairy Sci. 2008;91:1391-402.
29. Lean IJ, LeBlanc SJ, Sheedy DB, Duffield T, Santos JEP, Golder HM. Associations of parity with health disorders and blood metabolite concentrations in Holstein cows in different production systems. J Dairy Sci. 2023;106:500-18.
30. Klein D, Flock M, Khol JL, Franz S, Stüger HP, Baumgartner W. Ultrasonographic measurement of the bovine teat: breed differences and the significance of the measurements for udder health. J Dairy Res. 2005;72:296-302.
31. Sandrucci A, Tamburini A, Bava L, Zucali M. Factors affecting milk flow traits in dairy cows: results of a field study. J Dairy Sci. 2007;90:1159-67.
32. Ellis KA, Innocent GT, Mihm M, Cripps P, Mclean WG, Howard CV, Grove-White D. Dairy cow cleanliness and milk quality on organic and conventional farms in the UK. J Dairy Res. 2007;74:302-10.
33. Magnusson M, Herlin AH, Ventorp M. Effect of alley floor cleanliness on freestall and udder hygiene. J Dairy Sci. 2008;91:3927-30
34. Jørgensen HJ, Nordstoga AB, Sviland S, Zadoks RN, Sølverø L, Kvitle B, Mørk T. Streptococcus agalactiae in the environment of 1001 bovine dairy herds-rewriting the textbooks? Vet Microbiol. 2016;184:64-72.
35. Fontaneli RS, Sollenberger LE, Littell RC, Staples CR. Performance of lactating dairy cows managed on pasture-based or in freestall barn-feeding systems. J Dairy Sci. 2005;88:1264-76.
36. Goldberg JJ, Wildman EE, Pankey JW, Kunkel JR, Howard DB, Murphy BM. The influence of intensively managed rotational grazing, traditional continuous grazing, and confinement housing on bulk tank milk quality and udder health. J Dairy Sci. 1992;75:96-104.
37. AbuGhazaleh AA, Felton DO, Ibrahim SA. Milk conjugated linoleic acid response to fish oil and sunflower oil supplementation to dairy cows managed under two feeding systems. J Dairy Sci. 2007;90:4763-9.
38. Bargo F, Muller LD, Delahoy JE, Cassidy TW. Performance of high producing dairy cows with three different feeding systems combining pasture and total mixed rations. J Dairy Sci. 2002;85:2948-63.
Author notes
jmorales@inia.org.uy
Additional information
Transparency
of data: Data not available: The data set that supports the
results of this study is not publicly available.
Author contribution statement: Jéssica Tatiana Morales-Piñeyrúa conceived
and designed the study, collected the data, contributed to data analysis and
discussion, and wrote the paper. Pablo Ernesto Bobadilla contributed by
performing the analysis, discussing the results, and writing the paper. Ignacio
Alcántara contributed by performing the analysis. Elena de Torres contributed
by conceiving and designing the study, analyzing the data, discussing it, and
writing the paper.
Editors: The following editor approved this article:
Laura Astigarraga (https://orcid.org/0000-0002-7696-7539) Universidad de la
República, Facultad de Agronomía, Montevideo, Uruguay
Alternative link
https://agrocienciauruguay.uy/index.php/agrociencia/article/view/1150/1441 (pdf)









