INTRODUCTION
Ragweed (Ambrosia artemisiifolia L.) is a malicious invasive weed in Europe (Smith et al., 2013; Agnew et al., 2018; Bonini et al., 2018) and Asia (Fukano and Yahara, 2012), and has become a real disaster for the southern regions of Russia. The species was brought to the Old World from North America in 1873 with red clover seeds.
In Russia, ragweed was first discovered in 1918 by the botanist S. G. Kolmakov near Stavropol. At about the same time, a quarantine plant was discovered in Krasnodar Krai. In the Rostov region, the first foci of ragweed appeared in the early fifties and in Primorsky Krai - in the early sixties of the last century.
Ragweed is one of the most dangerous weeds that cause an allergic reaction in humans. Due to its biological plasticity and the absence of natural enemies, it
is widely introduced into plant communities around the world (Chen et al., 2018). Ragweed strongly inhibits cultivated
plants (Baysinger and Sims, 1991; Harrison et al., 2001), dries up the soil, sharply reduces soil fertility,
its pollen causes massive allergic diseases in humans (Basset and Crompton,
1975; Smith et al., 2013; Esipenko et al., 2016; Gentili et al., 2019). The absence of natural enemies of this weed causes
its uncontrolled invasive development and spread. Then, it is mainly controlled by mechanical and chemical
means.
Agrotechnical methods could not
stop the large-scale invasion of ragweed both in Russia and in other countries.
Chemical herbicides are also often ineffective against this dangerous plant due
to the development of resistance (Barnes et
al., 2017; Nagy et al., 2017). Currently, the North
Caucasian region and Primorsky Krai are the zones of
the most remarkable development and spread of the weed. Due to global warming, there is a
rapid expansion of the territories of invasion: there is a vertical acclimatization of the weed,
which gradually moves northward (Scalone et al., 2016; Lake et al., 2017). Full maturation of
ragweed seeds is observed in the regions of central Russia, which was not
previously noted. As early as 20 years ago, the total area
of the land infested with ragweed in Russia significantly exceeded 6 million
hectares (Moskalenko, 2001).
From 1967 to 1979, more than 30 species of natural enemies of ragweed were selected in Canada and the United States and introduced to the territory of the former USSR (Kovalev and Runeva, 1970; Goeden et al., 1995).
They include four species of phytophagous insects from natural complexes of North America: TarachidiacandefactaHubn, Euaresta bella Loew, Brachytarsus tomentosusSay, and Zygogrammasuturalis F., which were introduced and released in the North Caucasus (Kovalev and Runeva, 1970; Kovalev and Samus', 1972; Kovalev, 1981). In the first years after acclimatization, the abundance of these species was very low, and they were considered unpromising for the biological control of ragweed.
It is known that two herbiphages have acclimatized in Russia: the ragweed leaf beetle and the ragweed moth (Matishov et al., 2011).
For some years after the introduction, the ragweed moth population was in depression. Since 2000, its population density in the south of Russia began to grow. In some years, the number reached 10 ind./m2, which was sufficient for a 30% suppression of the weed in the phase of 6-8 true leaves.
For more effective weed control, a larger amount of herbiphage is required than it can be developed in the natural conditions of the Krasnodar Territory. Therefore, additional breeding of T. candefacta in artificial conditions (laboratory or specialized biofactory) is necessary.
This work aims to improve the method of
seasonal colonization of the ragweed moth T. candefacta, based on the early mass
cultivation of the herbiphage on an artificial
nutrient medium and its dispersal at the beginning of the growing season of
ragweed.
MATERIALS AND METHODS
he development of a seasonal colonization method includes:
- a collection of caterpillars of T. candefacta of III – V age in the autumn period, with their subsequent rearing in the laboratory on the ANM until pupae are formed;
- placement of pupae for storage in vessels with calcined river sand at a temperature of + 3 + 5 ° C, relative humidity of 70-80%, where they are stored until February;
- reactivation of pupae in a climatic chamber at temperatures up to 23-25 ° C.
The butterflies were kept at a
temperature of 23-24 ° C., a day length of 18 hours, and an air humidity of
70-80% in glass jars. Several pairs of butterflies were placed in each jar and fed with a 5% aqueous solution of
sugar. Filter paper folded like an accordion for egg- laying was placed inside
the jar. In 3-4 days after the emergence of butterflies, egg-laying begins,
which lasts 4-6 days. The eggs were incubated at 23-25 ° C and 60-70% humidity.
The hatched caterpillars were placed on the ANM, where they were reared until age III, and then released on young ragweed plants during the germination period. Mass rearing of the ragweed moth on ANM was carried out in cages with the size of 30x40 cm, which were covered with a perforated plastic wrap to prevent the food from drying out.
When developing artificial nutrient media, the caterpillars of the experimental and control variants were kept in Petri dishes at a temperature regime (+ 25-26 ° C), relative air humidity of 70-80%, and natural light; the food was changed once a day. Experiments were performed in six replicates, with 4-5 hatched individuals in each replication. Caterpillars of older ages, due to inherent cannibalism, were kept individually in a separate Petri dish.
The artificial feed quality was assessed according to the biological indicators of the development of caterpillars I-II; III-V ages, the mass of caterpillars and pupae.
In experiments on the development of an artificial nutrient medium for breeding T. candefacta under laboratory conditions, the following composition was used as a basis: dry ragweed leaf, soybean meal, dry brewer's yeast together with B vitamins, gelatin, formalin, sorbic acid and water (Agasyeva et al., 2017). To improve the ANM, milk powder was added to its composition in 0.1 g, 0.3 g, 0.5 g, and 1.0 g. As a control, natural food was used - Ambrosia artemisiifolia L plants. The composition was also tested with the addition of a vitamins mixture: riboflavin - 0.0002%, cyanocobalamin - 0.0005%. The aqueous solution of the mixture was used at a concentration of 0.3; 0.5; 1.0; 1.5; 2.0%. ANM, which does not contain vitamins, served as a control.
Fifteen minutes before preparing the medium, the
required amount of gelatin was weighed and soaked in cold distilled water. The
listed components were dosed and mixed. After mixing all the ingredients, we
added gelatin, formalin with sorbic acid, and water, then the medium was brought to a homogeneous
consistency (Agasyeva et al., 2017).
To study the effectiveness of the colonization
method, which consists of developing starting colonies of the ragweed moth and releasing them into natural conditions, caterpillars of II-III
ages grown on ANM were uniformly released into the centers of seedlings of Ambrosia artemisiifolia L. in the third part of April. The experiment was carried out during 2016 to 2019 on the basis of a ten-field grain- row crop rotation at the Federal
Research Center of Biological Plant Protection (Central zone of Krasnodar Krai,
Russia). The area of the site where the foci with ragweed plants were located and the release of T. candefacta was
carried out was two hectares.
RESULTS
The nutritional requirements of
insects encompass the complex of chemical and physical
characteristics of the food that ensure their normal growth and development.
The former includes proteins, carbohydrates, lipids, vitamins, mineral salts,
water, various phage stimulants, etc. The latter is reduced to the structure and consistency of food,
which determine its av
The nutritional requirements of insects encompass the complex of chemical and physical characteristics of the food that ensure their normal growth and development. The former includes proteins, carbohydrates, lipids, vitamins, mineral salts, water, various phage stimulants, etc. The latter is reduced to the structure and consistency of food, which determine its availability and suitability for an insect.
We included milk powder as a protein and amino acids source in the ANM composition. The results given in table 1 indicate the possibility of growing the ragweed moth caterpillars, before pupae formation, on a medium containing milk powder as a source of proteins. As a result of growing the ragweed moth caterpillars on the developed ANM, the nutrient medium’s composition effectively improves the main biological indicators of the growth and development of the bioagent (Table 2).
Table 1. The effectiveness of feeding the ragweed moth caterpillars with the inclusion of powdered milk in the composition of the ANM
 *Note: there are no statistically significant differences according to Duncan's test at the 95% probability level between the
options marked with the same letter indices when compared within the columns.
*Note: there are no statistically significant differences according to Duncan's test at the 95% probability level between the
options marked with the same letter indices when compared within the columns.
|
Experiment option
|
Powdered milk,
g, 100 g of medium
|
The
number of caterpillars in the experiment,
|
Were fed till the age, %
|
Weight of pupae, mg
|
Average duration of development before
pupae formation, days.
|
|
ind.
|
III
|
IV
|
V
|
|
1
|
0,1
|
30
| 72,0a*
| 58,0 ab
| 52,0 a
|
38,7 c
|
28,0 b
|
|
2
|
0,3
|
30
|
72,0 a
|
60,0b
|
58,0 ab
|
36,7 b
|
27,3 ab
|
|
3
|
0,5
|
30
|
80,0 b
|
68,0 c
|
62,0 b
|
41,3 d
|
25,7 a
|
|
4
|
1,0
|
30
|
68,0 a
|
52,0 a
|
38,0 c
|
27,3 a
|
30,7 d
|
|
Control
|
-
|
30
|
96,0 c
|
88,0 d
|
72,0 d
|
48,7 e
|
20,7 c
|
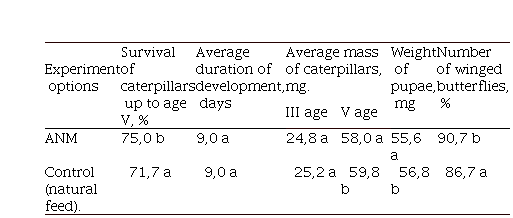
Table 2. Biological parameters of the growth and development of the ragweed moth (Tarachidia candefacta Hubn.) when grown on ANM of the original composition.
|
Experiment options
|
Survival of caterpillars up
to age V, %
|
Average duration
of development, days
|
Average mass
of caterpillars, mg.
|
Weight of pupae,
mg
|
Number of winged butterflies,
%
|
|
III
age
|
V age
|
|
ANM
|
75,0 b
|
9,0 a
|
24,8 a
|
58,0 a
|
55,6 a
|
90,7 b
|
|
Control
(natural feed).
|
71,7 a
|
9,0 a
|
25,2 a
|
59,8 b
|
56,8 b
|
86,7 a
|
According to our observations of the biological development of T. candefacta in the natural conditions of the Central zone of Krasnodar Krai, it was found that pupae overwinter, and butterfly flying is observed at the end of the first part of May. After mating, egg-laying is observed on the fourth day; eggs are attached to the leaves and stems of ragweed. Reproduction of moths is, on average, 300 eggs. One caterpillar can destroy 3-4 young ragweed plants with 3-4 true leaves, two caterpillars suppress the development of one ragweed plant that has reached the phase of eight true leaves, and ten caterpillars lead to the death of one plant in the budding phase (30-35 cm high). The offspring from one pair of ragweed moth butterflies within two generations can completely destroy ragweed in the early stages of development on an area of 1 m2. The average daily food requirement of 4th age caterpillars is found to be 60 mg.
DISCUSSION
Proteins and amino acids have a decisive influence on the growth and development of insects. They serve as the basis for building the insect's body. There is a direct relationship between the amount and quality of proteins in food and the fertility of phytophagous insects (Edel'man, 1972).
With the inclusion of 0.5 g of powdered milk per 100 g of the medium, the mass of pupae was 41.3 mg on natural food with 48.5 mg, and the average duration of development was 25.6 days and 20.5 days, respectively. These indicators show the advisability of including powdered milk in the composition of the ANM as a source of protein.
To activate the vital activity of the ragweed moth caterpillars and increase their viability, vitamins must be added to the artificial feed. They regulate the activity of enzymes and carbon metabolism, control tissue respiration and metabolism in general, and positively affect the growth and development of insects. It was found that the use of a complex of vitamins in the ANM improves the biological parameters of growth and development of the ragweed moth. The best results were obtained with a 1.5% concentration of vitamins. The viability of caterpillars was 85.3%, 39.6% higher than the control variant; the duration of insect development decreased by 3.8 days compared to with the control (ANM without vitamins).
The results obtained showed that the lack or excess of vitamins is likely to lead to metabolic disorders in caterpillars, which causes a decrease in the biological indicators of the growth and development of the herbiphage. By replacing wheat germ - the most commonly used ingredient in ANM by a number of researchers - with soybean meal (which has an optimal ratio of amino acids as a protein ingredient), we have obtained a high-quality medium balanced in terms of protein and amino acid composition.
Caterpillars of herbiphage grown on ANM were used to suppress ragweed on the experimental plot. Colonized caterpillars evenly crawled along with the ragweed shoots, fed there for two and a half weeks, and went into the soil for pupation. In the artificial reserves of the ragweed moth, the seedlings of the weed were almost destroyed, and the remaining ones lagged significantly in growth and development compared to the control plants, where the release of the release of the herbiphage was not carried out.
The first caterpillars of the new generation were observed at the places of introduction in late May and early June. After five days, their numbers averaged 2-3 caterpillars per one oppressed plant. By this time, the beginning of mass flying of the natural population of the ragweed moth was observed, which led to an intensive increase in the population density of the bioagent, which determines an artificial shift in the phenophase of herbiphage development and high biological efficiency against juvenile ragweed phases.
After butterflies mating, egg-laying is observed on the fourth day. The eggs are attached to the leaves and stems of ragweed. Reproductiveness of female moths was noted at the level of 200-300 eggs. As a result of the experiment, it was found that to suppress the growth of ragweed in the plant phase of 5-8 leaves by 50%, at least 20 ind./ m2 of T. candefacta caterpillars are required, which leads to inhibition of plant development and prevents the flowering process.
CONCLUSIONS
The possibility of mass breeding the ragweed moth on an artificial nutrient medium under laboratory and production conditions will allow early colonization of the ragweed moth caterpillars in the places where the first ragweed shoots appear.
A method of seasonal colonization of the ragweed moth was developed based on the early mass cultivation of herbiphage and its release at the beginning of the weed growing season. It is associated with an artificial shift in the phenophase of the ragweed moth, which makes it possible not only to suppress the weed in the early phases of development but also to control the number and useful activity of the herbiphage.
REFERENCES
Agasyeva, I.S., Fedorenko, E.V., Ismailov, V. Y. and Ermolenko S.A. 2017. Method for the production of a nutrient medium for breeding the ragweed moth Tarachidia candefacta Hubn. Patent of Russia No. 2628793, Bull. No. 24.
Agnew, M., Banic, I., Lake, I.R., Goodess, C., Grossi, C.M., Jones, N.R., Plavec, D., Epstein, M. and Turkalj, M. 2018. Modifiable Risk Factors for Common Ragweed (Ambrosia artemisiifolia) Allergy and Disease in Children: A Case- Control Study. International Journal of Environmental Research and Public Health 7: 1339. doi: 10.3390/ijerph15071339
Barnes, E.R., Knezevic, S.Z., Sikkema, P.H., Lindquist, J.L., Jhala, A.J. 2017. Control of Glyphosate-Resistant Common Ragweed (Ambrosia artemisiifolia L.) in Glufosinate-Resistant Soybean [Glycine max (L.) Merr]. Frontiers in Plant Science 8: 1455. doi: 10.3389/fpls.2017.01455
Basse, I.J. and Crompton, C.W. 1975. The biology of Canadian weeds Ambrosia artemisiifolia L. and A. psilostachya DC. Crompton Canadian Journal of Plant Science. 55: 463–476.
Baysinger, J.A. and Sims, B.D. 1991. Giant ragweed (Ambrosia trifida L.) interference in soybeans (Glycine max), Weed Science 3: 358–362
Bonini, M., Šikoparij, B., Skjøt, C.A., Cislaghi, G., Colombo, P., Testoni, C. and Smith M. 2018. Ambrosia pollen source inventory for Italy: a multi- purpose tool to assess the impact of the ragweed leaf beetle (Ophraella communa LeSage) on populations of its host plant. International Journal of Biometeorology 4: 597-608. doi: 10.1007/s00484-017-1469-z
Chen, K.W., Marusciac, L., Tamas, P.T., Valenta R. and Panaitescu, C. 2018. Ragweed Pollen Allergy: Burden, Characteristics, and Management of an Imported Allergen Source in Europe. International Archives of Allergy and Immunology 176: 163-180. doi: 10.1159/000487997
Esipenko, L.P. 2016. An adventive weed of American origin Ambrosia artemisiifolia L. as a source of allergy in the South of Russia and promising methods for its suppression, Proceedings of the Kuban Agrarian University. 58: 112-120.
Fukano, Y. and Yahara T. 2012. Changes in defense of an alien plant Ambrosia artemisiifolia before and after the invasion of a native specialist enemy Ophraella communa. Public Library of Science One 7: 11. doi:10.1371/journal.pone.0049114
Gentili, R., Asero, R., Caronni, S., Guarino, M., Montagnani, C., Mistrello, G. and Citterio, S. 2019. Ambrosia artemisiifolia L. temperature-responsive traits influencing the prevalence and severity of pollinosis: a study in controlled conditions. BMC Plant Biology 1: 155. doi: 10.1186/s12870-019-1762-6
Goeden, R.D., Palmer, W.A. and Scott R.R. 1995. Lessons learned from studies of the insects associated with Ambrosiinae. In North America in relation to the biological control of weedy members of this group, International Symposium on Biological Control of Weeds - Canterbury, New Zealand. pp. 565-573.
Harrison, S., Regnier, E., Schmoll, J., and Webb, J. 2001. Competition and fecundity of giant ragweed in corn. Weed Science. 2: 224-229.
Kovale, O. V. and Samus, V.I. 1972. Biology of the moth Tarachidia candefacta Hubn and the prospects for controlling ragweed, Agricultural biology. 2: 281-284.
Kovalev, O.V. 1981. Introduction and acclimatization of ragweed phytophages (Ambrosia L., Asteraceae) in the USSR, Aspects of general entomology 63: 31-32.
Kovalev, O.V. and Runeva, T.D. 1970. Tarachidia candefacta Hubn. (Noctuidae, Lepidoptera) moth is a promising phytophage in biological control of Ambrosia L (Compositae) weeds. Entomological Review. 49: 26-36.
Lake, I.R., Jones, N.R., Agnew, M., Goodess,C.M., Giorgi, F., Hamaoui-Laguel, L., Semenov, M.A., Solmon, F., Storkey, J., Vautard, R. and Epstein, M.M. 2017. Climate Change and Future Pollen Allergy in Europe. Environment Health Perspect. 3: 385-391. doi: 10.1289/EHP173
Matishov, G.G. 2011. Biological methods of controlling ragweed in anthropogenic phytocenoses of the south of Russia. pp. 139
Moskalenko, G.P. 2001. Quarantine weeds of Russia. M.: Rosgoskarantin, pp. 280
Nagy, E., Hegedűs, G., Taller, J., Kutasy, B. and Virág, E. 2017. Illumina sequencing of the chloroplast genome of common ragweed (Ambrosia artemisiifolia L.). Data Brief. doi: 10.1016/j.dib.2017.10.009
Scalone, R., Lemke, A., Štefanić, E., Kolseth, A.K., Rašić, S. and Andersson, L. 2016. Phenological Variation in Ambrosia artemisiifolia L. Facilitates Near Future Establishment at Northern Latitudes, Public Library of Science One 11. doi: 10.1371/journal.pone.0166510
Smith, M., Cecchi, L., Skjøth, C.A., Karrer, G. and Šikoparija, B. 2013. Common ragweed: a threat to environmental health in Europe. Environment International 61: 115-26. doi: 10.1016/j.envint.2013.08.005







