1. Introduction
Faecal egg count (fec) is used in sheep as a management
tool to decide when to drench in order to reduce the parasite burden(1).
It is also used in sheep genetic evaluations, where selection for low fec is the aim(2). In the
latter case, fec recording should
be carried out when the parasite burden is large enough to allow the expression
of between animal variation. National and international protocols suggest
drenching or recording the whole group when a fec
range of 500 to 1000 is reached. It is also sought that the fraction of animals
with a fec of zero should be less
than 0.1(3)(4). In order to decide when to drench, or to record fec in the whole contemporary group in
the case of genetic evaluations, a random sample of animals is monitored for fec. Two questions arise: (i) what is
the necessary sample size to determine if average fec reached a value of 500 or 1000? and (ii) what is the
necessary sample size to determine that the fraction of animals with fec equal to zero is less than or equal
to 0.1?
Collecting faeces and recording fec is time consuming and costly. If the number of animals sampled is too large, time and money will be wasted. By contrast, if the number sampled is too small, the information gathered will be of no value, and the resources used would have been wasted. In order to determine the necessary sample size, one has to decide the accuracy with which to estimate the population parameters, based on the sample. Sample size will be the result of a balance between the desired accuracy of the estimate, and the effort and cost entailed in obtaining it.
Practical guides on internal parasite control recommend some target values. WormBoss(5), the Australian program for the control of internal parasites in sheep and goats, recommends sampling no less than 20 animals, whereas Fiel and others(6) in Argentina recommend a minimum of 10 animals, and “ideally”, 20. In Uruguay, Pereira(7) recommends a minimum of 15 animals and an average fec of at least 600 to 800. Also in Uruguay, Castells(8) recommends monitoring 15 to 20 animals, and recording the whole group when the average fec in the sample is greater than 500 and the animals with a record of zero are less than 20%.
In this paper we present a logical framework (theory and examples) to work out the sample size of monitored animals in order to decide when to drench or when to record fec in the whole contemporary group. The treatment of the subject matter follows the methodology given in statistical textbooks such as Freund(9), Ott and Longnecker(10), and Snedecor and Cochran(11).
2. General considerations
There are two main considerations we should make when determining the necessary sample size: (i) specify the “tolerable” error, that is, the desired magnitude of the confidence interval, and (ii) establish the confidence level with which to make the estimate.
If we specify a confidence interval that is too broad, the estimate of the mean (µ) will not be very informative. Similarly, a low level of confidence will probably result in an erroneous confidence interval that might not include µ. By contrast, if we establish a narrow confidence interval and a high level of confidence, the necessary sample size may be too large and difficult to justify in terms of time and cost. That said, what constitutes an appropriate degree of certainty?
In practice, a confidence level of 95% is often chosen. This has been generally adopted in agriculture because it may be argued that it is acceptable for biological variables related to production(12). In the long term it results in a probability of 1 in 20 of not including the parameter value of the population, a situation that may generally be considered acceptable for the type of work in question. The “tolerable” error depends on the context, namely, our knowledge about the implications of variability in the character under study. For example, we could establish a tolerable error of 200 or 400 for fec, implying confidence limits of 1000 ± 100 or ± 200, respectively. In the case of fraction of animals with zero fec, the tolerable error could be 0.02 or 0.04, corresponding to confidence limits of 0.1 ± 0.01 o ± 0.02, respectively.
3. Theory
Call µ and ȳ
the population and sample mean, respectively. If we take a sample of size n,
the standard error (S) of ȳ is equal to: , where σ is the standard deviation of the
character in the population, Φ = n/N (fraction sampled)
and N is the population size. When n is small relative to N
(say, Φ < 0.1) the fraction sampled may be ignored without fear of
incurring in any important error(11). This means that the standard
error of the mean in the sample is more dependent on the sample size than on
the population size. We ignore Φ in the derivations that follow but we
shall consider it later in relation to the correction for finite size of the
sampled population.
Three factors determine the confidence interval estimated for a population mean µ from a sample: (i) the desired confidence level (zα/2 value, i.e. 1.96 for 95%), (ii) the standard deviation of the character, and (iii) the sample size. We may infer the magnitude of the standard deviation from earlier samplings or from other studies of the character in question.
Assume we wish to estimate µ with a confidence interval (tolerable error) E. The confidence limits are ȳ ± L, where ȳ is the sample estimate of µ and L = E/2. For a confidence level of 95%, our estimate of µ is: . Thus, , where σ is the standard deviation of the character and n is the sample size. Rearranging, we obtain: . Consistent with what we earlier stated, sample size does not depend on the total number of animals in the population under study. We return to this point later in the paper in relation to small populations.
We treat the fraction of animals with zero fec as having a binomial distribution. Then, , where p and q are the proportion of animals with zero and greater than zero counts, respectively. Rearranging, we obtain: . Note that p, q and L may be expressed as a proportion or as a percentage, but when performing calculations, the same unit must be used for p, q and L.
4. Results from a few examples
4.1
Estimating mean fec
Our experimental records
of fec show that the standard
deviation is always of similar magnitude as the mean, and often somewhat
greater (~10 to 20%). This is consistent with the mean and standard deviation
values reported by Goldberg and others(13) for 8 to 12-month-old
sheep. Hence, the mean and standard deviation values of fec chosen in Table 1.
Table 1:
Necessary number of animals to be sampled to estimate fec with different confidence intervals, for a range of
population means and standard deviations
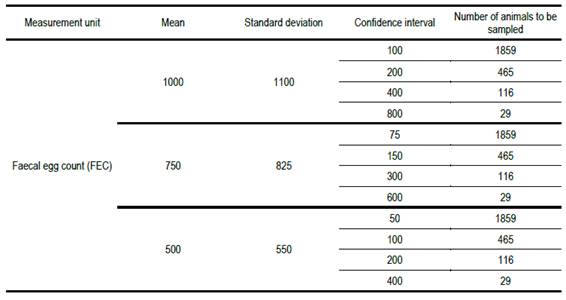
When the desired
confidence interval for the estimate of mean fec
is 10% of the population mean value, the required number of animals to be
sampled is prohibitively high. It is only when the confidence interval is 80%
of the population mean value that the necessary number of animals to be sampled
is near the number sampled in practice (20). A sample of 20 animals implies a
confidence interval of 96% of the population mean.
4.2 Estimating the fraction of animals with fec equal to zero
There are reports indicating that a large fraction of internal parasites in a flock may reside in a relatively small fraction of the animals(3). This may be the reason why in the implementation of internal parasite control programs there is sometimes a requirement that the fraction of animals with fec equal to zero should not exceed 0.10, recorded with a precision of 100. The fraction of animals with fec equal to zero is calculated from the same sample used to estimate mean fec.
Our experimental records show that for mean fecs of 1000, 750 and 500, the corresponding fraction of animals with a fec of zero is around 0.05, 0.10 and 0.15, respectively. The values chosen in Table 2 to calculate the necessary number of animals to be sampled are consistent with this experience.
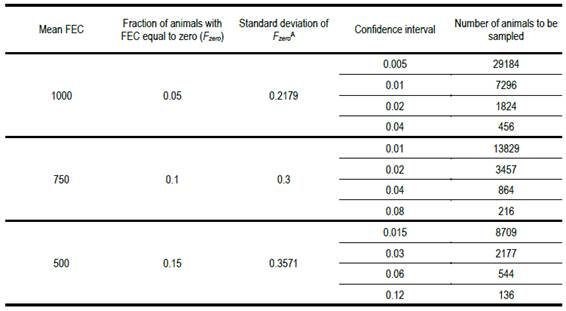
Table 2:
Number of animals to be recorded for fec
to estimate the fraction with zero count with different confidence intervals,
for a range of population means and standard deviationsA -
Calculated as (pq)0.5, where p is the fraction of
animals with fec of zero
 A - Calculated as (pq)0.5,
where p is the fraction of animals with fec of zero
A - Calculated as (pq)0.5,
where p is the fraction of animals with fec of zero
When the confidence
interval with which we wish to estimate the fraction of animals with a zero fec is 10% of the population mean
value, the necessary number of animals to be sampled is extraordinarily high.
It is only when the confidence interval is 80% of the mean and the fraction of
animals with a zero fec is 0.15,
that the necessary number of animals to be sampled nears something that may be
practicable. Note, however, that even in that case, the number is seven times
greater than what the guidelines state. The recommended number (20 animals
sampled) implies a confidence interval more than twice as large as the population
mean. The recommended practice is questionable. The results in Table 2 show
that it is not possible to adequately estimate the fraction of animals with a
zero fec from a sample of animals
smaller than 100.
5. Discussion and conclusions
In the calculations leading to the results presented in Tables 1 and 2 we ignored the correction for small populations mentioned by Snedecor and Cochran(11). These authors recommend making the correction when the fraction of sampled animals is greater than 10% of the total. In such cases the value of n should be corrected as follows: , where nc is the number of animals to be sampled corrected for finite size, and Φ is the fraction sampled. Snedecor and Cochran(11) indicate that when the sampled fraction is smaller than 10% the correction is unnecessary.
Table 1 shows that the necessary number of animals to be sampled, for a range of confidence intervals of 10 to 80% of the mean, is greater than the greatest number currently recommended of 20. The same result is obtained for different mean fecs because we assumed confidence intervals proportional to the mean. The calculations also assume that fec is normally distributed or approximately so. We know that, particularly in small populations, that assumption is unlikely to be satisfied. This would make the problem even more serious.
As an example, using round numbers, for the case in Table 1 where the necessary number of animals to be sampled is 116 animals, if the total number in the group was 232, the correction would result in nc = 116 / (1 + 0.5) = 77,3 ~ 77, smaller than 116, but still much greater than the currently recommended sample size. If the most commonly encountered situations were defined, tables could be developed as a guide for populations in which the fraction sampled was greater than 10% of the total.
Table 2 shows that the necessary number of animals to be sampled to estimate the fraction with a zero fec is very high, even if we assume a fraction 0.15 have that value. This indicates that a sample of 20 or fewer animals is far from satisfactory. Even in the most favourable case (p = 0.15 and a confidence interval of 80% of the population mean) the necessary number of animals to be sampled is almost seven times greater than 20, the maximum number currently recommended.
Jointly considered, Tables
1 and 2 show that the current guidelines regarding number of animals to be
sampled for fec suggest a number
much smaller than that emerging from the present statistical study. Internal
parasites in sheep have been mainly controlled by treatment with anthelmintics.
This strategy has not been entirely successful. Among other problems, the
parasites have developed resistance to some chemical groups, making treatments
less effective, or in extreme cases, totally ineffective. At best, decisions
about when to drench are made on the basis of fec
performed in a sample of 10 to 20 animals. This sample size is well below the
recommendations emerging from our statistical study of the problem. It is
difficult to avoid the conclusion that the inappropriate (much smaller than
required) sample size on which decisions about when to drench are made is at
least partly responsible for the failure to control internal parasites in
grazing sheep. Given the insufficient sample size currently used, we recommend
that guidelines should be revised and that, based on statistical criteria, they
should be reformulated.
Acknowledgments
Dr. Daniel Castells was the first in
drawing our attention to the problem we address in this paper.
References:
1. Court J, Webb Ware J, Hides S. Sheep farming for meat and wool: disease management. Collingwood: CSIRO; 2010. 336p.
2. Stear MJ. Breeding for resistance to nematode infections. In: Bishop SC, Axford R, Frank N, Owen JB, editors. Breeding for disease resistance in farm animals. 3rd ed. Wallingford (UK): CABI; 2010. p. 279-94.
3. Castells D. Adaptación de genotipos a ambientes adversos: resistencia genética de los ovinos a parásitos gastrointestinales. Agrociencia Uruguay. 2005;9(1-2):587-93.
4. SUL. Manual práctico de producción ovina. Montevideo: SUL; 2018. 221p.
5. WormBoss. Australia's sheep and goat worm control resource (Internet). Australia: Sheep CRC; (c2020; cited 2020 May 11). Available from: https://bit.ly/3czBmR7.
6. Fiel CA, Steffan PE, Ferreyra DA. Diagnóstico de las parasitosis más frecuentes de los rumiantes: técnicas de diagnóstico e interpretación de resultados. Tandil (AR): Abad Benjamin; 2011. 131p.
7. Pereira D. Revalorizando una herramienta fundamental: el análisis coprológico. Lana Noticias. 2002;(130):21-3.
8. Castells D. Evaluación de resistencia genética de ovinos Corriedale a los nematodos gastrointestinales en Uruguay: heredabilidad y correlaciones genéticas entre el recuento de huevos de nematodos y características productivas (master’s thesis). Montevideo (UY): Universidad de la República, Facultad de Veterinaria; 2008. 54p.
9. Freund JE. Modern elementary statistics. 3rd ed. Englewood Cliff: Prentice-Hall; 1967. 442p.
10. Ott RL, Longnecker M. An introduction to statistical methods and data analysis. Pacific Grove: Thomson Learning; 2001. 1152p.
11. Snedecor GW, Cochran WG. Statistical methods. Ames: Iowa State University Press; 1971. 593p.
12. Lahoz R, Ortega J, Fernández C. Métodos estadísticos en biología del comportamiento. Madrid: Editorial Complutense; 1994. 232p.
13. Goldberg V, Ciappesoni G, De Barbieri I, Rodríguez A, Montossi F. Factores no genéticos que afectan la resistencia a parásitos gastrointestinales en Merino en Uruguay. Producción Ovina. 2011;(21):1-11.
Notes
Author contribution statement: All authors (WB, ALS and RWP) of the paper
entitled “Sample size needed to make decisions about measuring FEC in a
contemporary group of lambs” contributed in an equal manner to the development
and finalization of the manuscript.
Editor: The following editor approved this article.
Gabriel Ciappesoni ORCID: https://orcid.org/0000-0002-0091-3956
Instituto Nacional de Investigación Agropecuaria (INIA), Montevideo, Uruguay.
Alternative link
http://agrocienciauruguay.uy/ojs/index.php/agrociencia/article/view/206/303 (pdf)







