Introduction
For years it has been recognized that South American Camelids (CSAs) tend to have higher blood glucose concentrations than domestic ruminants, which is puzzling because CSAs, like other ruminants, are also fermenters; It is not possible to think that the carbohydrates in the diet escape from gastric microbial fermentation, so these species depend on gluconeogenesis to supply the necessary substrate to glucose-dependent tissues. Furthermore, in recent years, diseases have been identified in alpacas and llamas, which appear to be the result of abnormal carbohydrate metabolism. Some of these alterations resemble those of other animals, but others do not; these differences support the conclusion that CSAs process carbohydrates differently from other animals1.
Faced with a decrease in food consumption, the animal organism activates mechanisms that lead to the production of substrates that ensure the supply of energy to the vital organs, in order to ensure the survival of the animal, and, if the fast continues, the metabolism is changing both qualitatively and quantitatively2. During fasting, initially, the energy substrates derive from protein and fat degradation, but in the following phases, protein degradation slows down, maximizing lipolysis3. Gluconeogenesis is a metabolic pathway that is permanently active in ruminants, with propionic acid being the main precursor of glucose synthesis4. Likewise, it seems that alpacas depend on their digestive and metabolic adaptations to obtain, efficiently conserve energy to survive in environments where the available food varies in nutritional quality5.
Another important source of energy is amino acids, which are mobilized after blood hypoglycemia in order to support hepatic gluconeogenesis and, therefore, energy production, due to these conditions, glucose, amino acids are maintained, but fats, ketone bodies, are mobilized for energy production6. On the other hand, urea is the degradation product of amino acids, in ruminants, this urea can be recycled and return to the rumen through the saliva or the ruminal wall, once present in the rumen, it is reconverted into ammonia which can serve for the synthesis of amino acids by the ruminal microbiota, and these, in turn, as a source of energy after intestinal absorption4.
The breeding of CSA constitutes the main means of productive use of large extensions of natural pastures, in the high areas of the Andes where it is not possible to breed other domestic species or agriculture, the alpaca, efficiently converts natural vegetation of these environments into fiber and meat for human consumption7. However, the rearing conditions of the CSA, the seasonal variability of the nutritional resources determine that they have to go through nutritional limitations during the dry season and, in many cases, for prolonged periods of fasting8. The alpaca, like the other CSAs, are species that have evolved efficiently in environments with serious environmental limitations but are not yet well understood9. During fasting, these animals can use free fatty acids (FFA) and ketone bodies (KB) more effectively and do not need to depend entirely on the biochemical pathways of classical ruminants for their energy and glucose requirements10.
In one study, plasma glucose, creatinine, and urea concentrations in flames were reported to be higher than sheep and goats, but very low in ketone bodies (β-hydroxybutyrate)11. The high level of glycemia can be explained in part by a poor response to insulin or by reduced sensitivity of the tissue to insulin12. Furthermore, CSAs cover their glucose requirements through the gluconeogenesis process from the amino acids of the microbial protein and not from propionate, as occurs in ruminants9.
These characteristics imply the need for a dietary scheme based on the protein-fat ratio and not on the protein-carbohydrate ratio as in ruminants9. For this reason, the study was proposed with the objective of determining some variations in energy metabolism due to the influence of prolonged fasting in alpacas through serum determinations of glucose, total proteins, urea, and ketone bodies.
Materials and methods
The study was carried out at the
“La Raya” Research and Production Center of the National University of the
Altiplano-Puno (UNAP), located in the district of Santa Rosa, Melgar province, Puno department, Peru, at an altitude of
4200 m and between the geographic coordinates of 10°13'33” S and 20°57'12” W.
The center is located in the Puna region, with two
well-marked seasons: “rainy season” (from December to March) and "dry
season" (from April to March). The average rainfall is 932 mm/year and the
temperature varies between -7 ºC and 15 ºC. The pluvial precipitation and
temperature determine that the climate is of the humid cold type. The soil in
the area is dominated by the species Stipas, Festucas, Muhlenbergias,
Calamagrostis, and Distichias, which serve as food
for the extensive rearing of alpacas and llamas.
 Figure 1
Herd of two-year-old male alpacas (tuis)
Figure 1
Herd of two-year-old male alpacas (tuis)
The study was carried
out in May 2018. The analysis of the samples was carried out in the
Biochemistry Laboratory of the Faculty of Veterinary Medicine and Zootechnics
of the UNAP.
 Figure 2
Group of fasting animals
Figure 2
Group of fasting animals
Twenty-two-year-old
male alpacas of the Huacaya breed were used in
apparent good health, with an average live weight of 47.7 kg. The 20 animals
were randomly distributed in two treatments of 10 animals each (experimental
and control), which were kept in pens built with mesh and wooden posts with an
area of 25 m2. The experimental group remained confined during the
five days that the experiment lasted, without access to any type of food except
water and without prior accustoming period; meanwhile, the control group
animals were grazed together with the other animals, consuming the natural
pastures of the area. The animals were provided by the Institute for Research and
Promotion of South American Camelids (IIPC) of the UNAP.
 Figure 3
Animals of the control group
Figure 3
Animals of the control group
Blood samples were taken at 0, 24, 48, 72, and 96 h after the start of the experiment (hour 0 from 06:00 h) from all the animals. The samples in the control group were obtained before their departure to the grazing fields. Blood (5 mL) was obtained from the jugular vein in tubes without anticoagulant, and it was transferred to the laboratory in a refrigerated box. The serum was obtained by centrifugation at 1600 g for 10 min, and it was decanted into cryogenic vials for its conservation at -20 ºC until its processing. For the use of animals and obtaining blood samples, we had the authorization of the Ethics Committee of the Faculty of Veterinary Medicine and Zootechnics of the National University of the Altiplano.
The determinations of glucose, total proteins, and urea in the blood serum were carried out by colorimetry-spectrophotometry using reagents from Wiener Lab®. The determination of ketone bodies was made semi-quantitatively using Siemens ® Multistix 10 SG (Bayer) test strips that detect the presence of acetoacetate with a sensitivity of 5 to 10 mg/dL and reproducibility of 95% depending on the insert for use on the package.
The experiment was conducted in a randomized complete block design with sub-sampling (nested), considering as treatment the groups (experimental and control) and blocks at the sampling hours (0, 24, 48, 72, and 96 h). Duncan's test of significance13 was used for the comparison of means of the main effects (groups and sampling times) and for simple effects the LS Means of the SAS v. 9,114.
Results
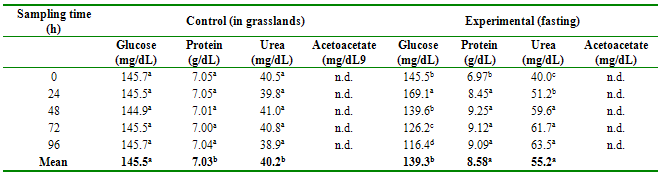
The concentrations of
glucose, protein, urea, and acetoacetate per group according to the time of
sampling (fasting) are shown in Table 1. Glucose levels were lower in fasting
animals compared to non-fasting animals (p≤0.05); and there are differences
between the sampling hours (p≤0.01) for the case of fasting animals, but not
for the control group animals. In contrast, protein and urea levels are higher
in fasting animals than in the control group (p≤0.01), as well as between
sampling days for fasting animals, but not for their controls (p≤0.01). Ketone
concentration readings were negative for both treatments (not detected).
Table 1
Biochemical profile
in blood serum of alpacas with and without fasting
n.d.: No detected
|
Sampling time
(h)
|
Control (in grasslands)
|
Experimental (fasting)
|
| |
Glucose
(mg/dL)
|
Protein
(g/dL)
|
Urea
(mg/dL)
|
Acetoacetate
(mg/dL9
|
Glucose
(mg/dL)
|
Protein
(g/dL)
|
Urea
(mg/dL)
|
Acetoacetate
(mg/dL)
|
|
0
|
145.7ª
|
7.05ª
|
40.5ª
|
n.d.
|
145.5b
|
6.97b
|
40.0c
|
n.d.
|
|
24
|
145.5ª
|
7.05ª
|
39.8ª
|
n.d.
|
169.1ª
|
8.45ª
|
51.2b
|
n.d.
|
|
48
|
144.9ª
|
7.01ª
|
41.0ª
|
n.d.
|
139.6b
|
9.25ª
|
59.6ª
|
n.d.
|
|
72
|
145.5ª
|
7.00a
|
40.8ª
|
n.d.
|
126.2c
|
9.12ª
|
61.7ª
|
n.d.
|
|
96
|
145.7ª
|
7.04ª
|
38.9ª
|
n.d.
|
116.4d
|
9.09ª
|
63.5ª
|
n.d.
|
|
Mean
|
145.5a
|
7.03b
|
40.2b
| |
139.3b
|
8.58a
|
55.2a
| |
| | | | | | | | | |
Discussion
The increase in glucose 24 h after fasting coincides with that reported by Wensvoort et al.10 who indicate that under fasting conditions the concentration of blood glucose increases in llamas, camels, and sheep. For their part, Tadich et al.15 confirmed that the increase in blood glucose in the first moment is mainly due to stress and not to the effect of fasting, since the initial hypoglycemia acts as a catecholamine-releasing factor, promoting glycolysis and gluconeogenesis, during stress, there is an increase in cortisol, which in turn is hyperglycemic. Similarly, Crossley et al.16, when evaluating the adaptive physiological response of the alpaca to the change of environment, found that the blood glucose concentration increases, a fact that is attributed to the stress caused by fasting, confinement, and taking samples.
The average glucose at the beginning of the experiment is within the range established as normal of 112-260 mg/dL by Siguas et al.17, and slightly lower than those reported by Kiani et al.11 and Al-Rehaimi et al. 18 who found glucose averages of 127.8 and 138.0 mg/dL in llamas and camels, respectively, differences that are attributed to the species. However, it is necessary to indicate that glycemia in alpacas, as in other camelids, is higher than other ruminants (cow, sheep, and goats) as reported by Kiani et al.11 This physiological hyperglycemia in alpacas is also due to the poor response of insulin and the low concentration of insulin in the serum, proposed by Cebra et al.1.
Glucose levels decrease after 24 h, which agrees with Albero et al.2, who indicates a rapid decrease in glycemia, but that after 48-72 h of fasting there is a slow decline until stabilizing. It is pointed out that, in essence, all the glucose available to ruminants, regardless of the diet, comes from gluconeogenesis, the most important precursor of glucose being propionic acid, and added to the constant gluconeogenesis, ruminants also cover their needs for glucose through effective conservation3.
The serum protein level at 0 hours (6.96 g/dL) coincides with the reports of Oblitas et al.19 who report an average concentration of 6.6 g/dL. In animals deprived of food, total protein levels rise after 24 h. However, this increase is not due to a real increase in proteins in the blood plasma, but rather to dehydration of the individual20. Other authors point out that in fasting, the kidney becomes the main source of water elimination, observing that in the first 5 to 10 days a strongly negative water balance is observed, even decreasing the density of urine21.
The serum urea concentration at the beginning of the experiment (40.25 mg/dL), is within the range reported by Siguas et al.17 (32.2 and 89.8 mg/dL). Likewise, it is similar to the value reported by Kiani et al.11 who determines uremia of 40.2 mg/dL for llamas.
Urea levels increase as the days of fasting pass. According to Bustamante22, when stressors are perceived, a cascade of events begins, most of them related to the hypothalamic-pituitary-adrenal cortex axis, which finally leads to the release of corticosteroids with certain physiological effects, including hepatic gluconeogenesis catalyzing amino acids and proteins and, therefore, a rise in urea concentration values. For their part, Chowdhury & Ørskov23 indicate that when fasting worsens in ruminants, glucoreceptors in the hypothalamus are stimulated, which stimulates the adrenal medulla to increase adrenaline secretion, which, in turn, activates glycogenolysis, lipolysis, gluconeogenesis Therefore, there will be greater use of intermediaries to produce energy, resulting in increased urinary nitrogen excretion during fasting. Likewise, Wensvoort et al.10 indicate that the increase in urea levels during fasting is a sign of an increase in amino acid oxidation to supply glucose precursors through gluconeogenesis.
Ketogenesis is likely to occur in longer periods of fasting, which is why its presence was not detected in the present study. The result agrees with Tadich et al.15, who also found no variations in ketone bodies (β-hydroxybutyrate) in the fasting of steers during transport to the slaughterhouse, possibly due to the use of the circulating pool of β-hydroxybutyrate provided by the rumen. For their part, Wensvoort et al.10 indicate that camelids could have the capacity to supply enough metabolites for the maintenance of the citric acid cycle and that they could use less or other metabolites in relation to other ruminant species. In this regard, it is pointed out that during the first days of fasting, the animal's energy reserves and gluconeogenesis serve to provide energy to the animal, and ketogenesis is activated when these reserves are depleted.24
Chandrasena et al.25 found plasma concentrations of 0.089 and 0.148 mg/dL of β-hydroxybutyrate and acetoacetate in camels, which are approximately 33 and 4 times lower than for the respective values in sheep, maintained under the same conditions, suggesting that the ketogenesis in camels is very low, a fact that would also be occurring in alpacas since at 96 h of fasting ketone bodies remain undetectable. Similarly, Kiani et al.11 concluded that levels of β-hydroxybutyrate in llama blood were extremely low for a ruminant animal, such as that found in alpacas in the present study. Thus, it is likely that the metabolism of ketone bodies in the alpaca shows variations with respect to that of other ruminants.
Cited literature
1. Cebra CK, Tornquist
SJ, Van Saun RJ, Smith BB. Glucose tolerance
testing in llamas and alpacas. Am J
Vet Res 2001;62(5):682-6. DOI: https://doi.org/10.2460/ajvr.2001.62.682
2. Albero R, Sanz A, Playán J. Metabolismo en
el ayuno. Endocrinol Nutr
2004;51(4):139-48. DOI: https://doi.org/10.1016/S1575-0922(04)74599-4
3. Cunningham J. Fisiología Veterinaria. Tercera ed. Madrid, España: ELSEVIER;
2003. DOI: https://doi.org/10.1016/B978-84-9022-317-8/00056-7
4. Dukes HH. Dukes´phisiology of domestic animals.
13th ed. John Wiley & Sons, Inc.; 2015.
5. Lund KE, Milton JTB,
Maloney SK, Blache D. Alpacas fed calcium propionate seem to moderate their
energy intake. J Anim Physiol Anim
Nutr (Berl). 2014;98(6):1088-94. DOI: https://doi.org/10.1111/jpn.12180
6. Mompeán O, Manjón Collado MT, Pérez Sánchez
A, Pérez Huertas R. Fisiopatología del ayuno corto y prolongado y del estrés. Rapd Online 2009; 32(1): 45-58.
7. Quispe EC, Rodríguez TC, Iñiguez LR, Mueller
JP. Producción de fibra de alpaca, llama, vicuña y guanaco en Sudamérica. Anim Genet Resour Inf 2009;45:1-14. DOI: https://doi.org/10.1017/s1014233909990277
8. San Martín F, Van
Saun RJ. Applied digestive anatomy and feeding
behavior. In: Cebra C, Anderson D, Tibary A, Van Saun R, Johnson LR, editors.
Llama and alpaca care: Medicine,
surgery, reproduction, nutrition, and herd health. Philadelphia: Elsevier; 2014. p. 51-8. DOI: https://doi.org/10.1016/B978-1-4377-2352-6.00008-0
9. San Martín F. Adaptación nutricional y
metabólica de los camélidos sudamericanos. En: Aranibar Aranibar
MJ, Urviola Sánchez JM, editores. VII Congreso mundial en
camélidos sudamericanos. Libro de resúmenes: 28, 29 y 30 de octubre
de 2015. Facultad de Medicina Veterinaria y Zootecnia [Internet]. Puno: Universidad Nacional del Altiplano; Corporación
Meru EIRL; 2015. [citado 3 de
mayo de 2019]. p. 45. Recuperado a partir de: file:///C:/Users/usuario/Downloads/LibroResumenes%20(1).pdf
10. Wensvoort J, Kyle D, Ørskov E, Bourke D. Biochemical
adaptation of camelids during periods where feed is withheld. Rangifer
2001;21(1):45-8. DOI: https://doi.org/10.7557/2.21.1.1527
11. Kiani A, Alstrup L, Nielsen MO. Differential metabolic and
endocrine adaptations in llamas, sheep, and goats fed high- and low-protein
grass-based diets. Domest Anim
Endocrinol 2015;53:9-16. DOI: https://doi.org/10.1016/j.domaniend.2015.03.006
12. Elmahdi E, Sallmann HP, Fuhrmann H, von Engelhard W, Kaske M.
Comparative aspects of glucose tolerance in camels, sheep, horses and ponies. Comp Biochem Physiol A Physiol
1997;118A(1):147-51. DOI: https://doi.org/10.1016/s0300-9629(96)00449-5
13. Duncan DB. Multiple Range and Multiple F Tests. Biometrics. 1955;11(1):1-42. DOI: https://doi.org/10.2307/3001478
14. Inc. SI. SAS/STAT® 9.1 User’s Guide. NC: SAS
Institute Inc.; 2004.
15. Tadich N,
Gallo C, Echeverría R, van Schaik G. Efecto del ayuno durante dos tiempos de
confinamiento y de transporte terrestre sobre algunas variables sanguíneas
indicadoras de estrés en novillos. Arch Med Vet 2003;35(2):171-85.
DOI: https://doi.org/10.4067/S0301-732X2003000200005
16. Crossley JC, Marin
MP, Ferrando G, Raggi LA. Modificaciones adaptativas de algunas
constantes fisiológicas de alpaca (Lama
pacos) sometidas a cambios de ambiente. Arch Zootec 1994;43 (163):215-23.
17. Siguas O,
Paucar R, Olazabal J, San Martín F, Vélez V. Valores bioquímicos sanguíneos en
alpacas en dos épocas del año en condiciones de Huancavelica: Aportes al perfil
metabolico de la especie. Arch Latinoam Prod Anim [Internet]. 2007
[citado 5 de
octubre de 2019];15 (Suppl. 1):
496-497.
Recuperado a partir de: https://www.researchgate.net/publication/284411697_Valores_bioquimicos_en_alpacas_en_dos_epocas_del_ano_en_condiciones_de_Huancavelica_aportes_al_perfil_metabolico_de_la_especie
18. Al-Rehaimi AA, Al-Ali AK, Mutairy AR, Dissanayake AS. A
comparative study of enzyme profile of camel (Camelus dromedarius) hump and sheep (Ovis aries) tail tissues. Comp
Biochem Physiol 1989;93B(4):857-8. DOI: https://doi.org/10.1016/0305-0491(89)90057-6
19. Oblitas GF, Pedrozo PR, Wittwer MF, Böhmwald
H, Ludwing H. Valores sanguíneos en alpacas (Lama pacos)
reintroducidas en el sur de Chile. Vet Méx 1998;29(4):411-4.
20. Hervás
Abad E, Sánchez Polo MT, García Lopez PJ. Nutrición, ayuno y ejercicio [Internet]. Fundacion para la Formacion e Investigacion
Sanitarias de la Region de Murcia. 2012 [citado 3 de
mayo de 2019].
Recuperado a partir de: http://www.ffis.es/volviendoalobasico/tema_15_nutricin_ayuno_y_ejercicio.html
21. Saz Peiró P, Ortiz Lucas M. Fisiología y
bioquímica en el ayuno. Med Natur 2007;1(1):10-9.
22. Bustamante H. Determinación del efecto de
diferentes tiempos de ayuno y transporte terrestre sobre algunas variables
sanguíneas indicadoras de estrés en bovinos en el período Otoño-Invierno [tesis
licenciatura]. [Valdivia]: Universidad Austral
de Chile; 2001.
23. Chowdhury SA, Ørskov ER. Implications of fasting on the
energy metabolism and feed evaluation in ruminants. J Anim Feed Sci 1994;3(3):161-9. DOI: https://doi.org/10.22358/jafs/69830/1994
24. Dukes HH. Dukes’ Physiology of domestic animals.
30o. (Reece W, ed.). Oxford: John Wiley & Sons, Inc.; 2015.
25. Chandrasena LG,
Emmanuel B, Hamar DW, Howard BR. A comparative study of ketone body metabolism
between the camel (Camelus dromedarius)
and the sheep (Ovis aries). Comp Biochem Physiol 1979;64(1):109-12.
DOI: https://doi.org/10.1016/0305-0491(79)90192-5
Notes
Funding Source: The study was financed
with the authors' own resources.
Conflicts of interest: he authors declare
they have no conflicts of interest with respect to the research, authorship and
/ or publication of this article.
Acknowledgements: We are grateful to the Institute for Research and
Promotion of South American Camelids (IIPC) of the Faculty of Veterinary
Medicine and Zootechnics of UNA-Puno for having provided us with the animals
for the study. Likewise, to the technicians Martín Chaiña
and Vicente Flores for their support in the construction of the facilities and
taking samples of the study.
Ethical aspects: The study had the
approval of the Ethics Committee of the Faculty of Veterinary Medicine and
Zootechnics of the National University of the Altiplano and the guidelines
established by this Committee were followed.
____________
ID of article: 079/JSAAS/2020
____________
Cite as: J.
Selva Andina Anim. Sci. 2020; 7(2):63-71.
___________
Editor's Note: Journal of the Selva
Andina Animal Science (JSAAS) remains
neutral regarding jurisdictional claims published on maps and institutional
affiliations.
Author notes
* Contact address: Faculty of Veterinary Medicine and Zootechnics. National University of the Altiplano-Puno. Dirección: Av. Floral 1153. Puno, Perú. Tel: +051-599430. Mobile: +51 95196540
Pedro Ubaldo Coila-Añasco E-mail address: pcoila@unap.edu.pe
Alternative link
http://www.scielo.org.bo/scielo.php?script=sci_arttext&pid=S2311-25812020000200003&lng=es&nrm=iso&tlng=en (html)









