



Artículos de investigación
Hydrolysis of esters and dialkyl malonates mediated by t-BuNH2/LiBr/alcohol/H2O
Hidrólisis de ésteres y malonatos de dialquilo con t-BuNH2/LiBr/alcohol/H2O
Pädi Boletín Científico de Ciencias Básicas e Ingenierías del ICBI
Universidad Autónoma del Estado de Hidalgo, México
ISSN-e: 2007-6363
Periodicity: Semestral
vol. 8, no. 16, 119-125, 2021

Abstract: An efficient, simple protocol for the ester hydrolysis and monohydrolysis of dialkyl malonates by .-BuNH./MeOH/H.O with or without LiBr is described. The method is of general applicability since alkyl esters including Me, Et, .-Pr, .-Bu, Bn and allyl esters have been successfully cleaved to give the free carboxylic acids with excellent yields. The scope of the procedure was explored for the deprotection of a series of aliphatic, aromatic, unsaturated monoesters and dialkylmalonates. The reactions are, in general, very clean with really high yields, and no side products have been isolated. The selectivity of the method was demonstrated by the hydrolysis of esters in the presence of an .-Boc protecting group.
Keywords: Ester hydrolysis, monohydrolysis, dialkyl malonates, .-BuNH./LiBr/MeOH/H.O.
Resumen: Se describe una metodología eficiente y sencilla para la hidrólisis de ésteres y monohidrólisis de malonatos de dialquilo con el uso de .-BuNH./MeOH/H.O con y sin LiBr. El método es de aplicación general debido a que los ésteres de Me, Et, .-Pr, .-Bu, Bn y alilo se hidrolizan adecuadamente para dar los ácidos carboxílicos correspondientes con excelentes rendimientos. El alcance del procedimiento se exploró para la desprotección de monoésteres alifáticos, aromáticos e insaturados, así como de malonatos de dialquilo. Las reacciones son, en general, muy limpias, dan rendimientos altos y no se obtienen subproductos. La selectividad del método se demostró mediante la hidrólisis de ésteres en presencia de un grupo protector .-Boc.
Palabras clave: Hidrólisis de ésteres, monohidrólisis, malonatos de dialquilo, .-BuNH./LiBr/MeOH/H.O.
1. Introduction
Protection and deprotection of carboxylic acids with appropriate protecting groups are indispensable steps and commonly used transformations in the synthesis of amino acids, peptides, natural products and polyfunctional compounds (Greene, 1999; Pearson, 1999; Jarowicki, 1999; Jarowicki, 2001). Although methyl and ethyl esters are frequently encountered in organic synthesis because of their easy preparation, the t-butyl- and benzyl-derived esters are the most commonly used to protect carboxylic acids. On the other hand, i-propyl, allyl and prenyl esters are less frequently used.
For carboxylic acid deprotection a number of useful and reliable procedures are applied (Greene, 1999), including proton (Dhar, 2003) and Lewis (Marcantoni, 2001), acid- or base-catalyzed (Ilankumaran and Verkade, 1999), cleavage, SN2-type dealkylation (Parish and Miles, 1973) and hydrogenolysis (Mandal and McMurray 2007; Pearson, 1999; Jarowicki, 1999; Jarowicki, 2001; Salomon, 1994; Furlán, 1998; Lesutis, 1999; Salmar, 2006; Andrés, 2006; Lee, 2013; Bhattacharya, 2009; Ghosh and Aubé, 2011; Long and Jones, 2011; Koshikari, 2012; Mirgorodskaya, 2012; Hu, 2011; Nakamura, 2011; Ludwig, 2006; Durow, 2006; Yadav, 2002; Chee, 2001; Bartoli, 2000; Wu, 2000; Kaul, 2004; Kabalka, 2001; Andrés and de Rossi 2003; Um, 2007; Poisson, 2005; Morwick, 2006; Liotta, 1981; Li, 2008). Esterases are important biocatalysts that have found wide application in protecting groups chemistry (Kumar and Jolly 1999; Kadereit and Waldmann 2001; Fotakopoulou, 2007; Schmidt, 2005; Barbayianni, 2005; Bordusa 2002).
Recently a process that mimics enzymatic hydrolysis has been achieved on the surface of nanofibers formed by aggregation of peptide amphiphiles and peptide dendrimers (Delort, 2004; Guler and tupp, 2007). Many enzymatic hydrolytic processes involve metal cations that have been assumed to activate a water molecule or other nucleophilic groups such as alcoholic residues (e.g., serine, threonine) to attack electrophilic substrates such as carboxylic esters. Various types of transition metal complexes, including micellar and non-micellar systems, have been designed to mimic the functions played by the typical central ion, Zn(II), in Zn(II)-metalloenzymes. It is worth noting that useful catalysts for ester hydrolysis usually contain a transition metal chelated to bidentate or tridentate ligand amines such as 2(hydroxymethyl)pirydine, imidazole, and 1,10-phenantroline derivatives (Scrimin, 1992; Scrimin, 1994; Fujita, 1988; Weijnen and Koudijs, 1992).
Macrocyclic amines such as tris(2-aminoethyl)amine, tris(2-dimethylaminoethyl)amine, 1,5,9-triazacyclododecane, diethylenetriamine derivatives and 2-[bis(2-aminoethyl)amino]ethanol have also been used (Polyzos, 2007; Kimura, 1990; Xia, 2001). In addition, few examples have been reported concerning direct participation of amines such as Et3N, DBU, DBN, DIPEA, 2-aminobenzoate esters, 2-dimetylaminoethanol (DMAE), DMF, imidazole and N,N-diarylamonium pyrosulfate in the ester hydrolysis (Fife, 2002; Seebach, 1991; Barton, 1973; Kirsh and Jencks, 1964; Mattsson, 2007; Giusti, 2014).
On the other hand, half esters of malonic acid are very important building blocks for the synthesis of a variety of significant pharmaceuticals and natural products (Niwayama and Cho, 2009; Niwayama, 2008; Iosub, 2010; Watanabe and Ishikawa, 1999; Bartoli, 2006; Billamboz, 2009). Although the classical method for their preparation consisting in monosaponification of symmetric diesters has been recently applied with high efficiency, other methods using enzymatic, Cs2CO3/EtOH, KF/DMF, monohydrolysis of unsymmetrical ethyl t-butyl malonate with CeCl3·7H2O-NaI and reaction of esters with LDA/CO2/THF have also been reported.
Even though numerous methods of ester hydrolysis have been reported in literature, there is an ongoing need to develop methods that require mild conditions especially for compounds with acid and base sensitive functionalities. In this paper, we focused on the applications of the methodology using t-BuNH2/alcohol/H2O with or without LiBr for the ester hydrolysis. So we wish to report our studies on the removal of various carboxyl protecting groups, such as Me, Et, i-Pr, t-Bu, Bn and allyl esters and monohydrolysis of dialkylmalonates using the t-BuNH2/alcohol/H2O system.
2. Experimental
General Procedure for the Ester Hydrolysis. To a solution of the corresponding ester or malonate ester (0.6 mmol) in MeOH (5 mL) or EtOH (5 mL) was added water (2 mL), t-BuNH2 (10 equiv, 0.635 mL) and, when appropriate, LiBr (3 equiv, 0.159 g) and the mixture was stirred under reflux for the appropriate time (Tables). After complete conversion, as indicated by TLC or 1H NMR spectroscopy of the reaction crude, the mixture was cooled to rt and diluted with EtOAc (50 mL). The organic phase was washed with saturated aqueous of NH4Cl (2 x 30 mL) and brine (2 x 30 mL). The water phase was acidified with 10% aqueous HCl to pH 1 and extracted with EtOAc (2 x 30 mL). The organic phases were dried over Na2SO4, filtered and concentrated in vacuum to give the corresponding carboxylic acid. The identity and the purity of the reaction products were established by their 1H NMR data by direct comparison with authentic samples.
3. Results and discussion
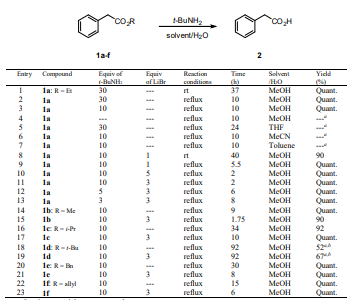
As indicated in Table 1, to screen for suitable reaction conditions our experiments were first conducted with readily available ethyl phenylacetate 1a as a model substrate by treating it with 30 equiv of t-BuNH2 in MeOH/H2O at room temperature during 37 h and desired phenyl acetic acid 2 was obtained in quantitative yield (Entry 1, Figure 1). The reaction progress was monitored until complete conversion by TLC analysis of the crude product showed the carboxylic acid 2 (baseline material) and a less polar compound corresponding to the methyl ester 1b derived by transesterification of 1a with methanol (Suárez-Castillo, O. R, 2008), which was gradually transformed into 2. After some trials, we found substantial reduction of reaction time (10 h) for the conversion of 1a into 2 with 10 equiv of base and treating the mixture under reflux, affording carboxylic acid 2 in quantitative yield (Entry 3).
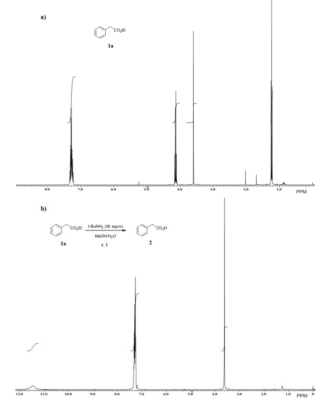
To confirm that t-BuNH2 facilitated the ester hydrolysis process, ester 1a was treated under reflux of MeOH/H2O in the absence of the base but no carboxylic acid 2 was detected after 10 h of reaction (Entry 4). For other solvents e. g. THF, MeCN and toluene no conversion of 1a into 2 was seen under reflux (Entries 5-7), showing that MeOH is critical for the hydrolysis reaction.
For ester hydrolysis we also used the t-BuNH2/MeOH/H2O system together with LiBr (Suárez-Castillo, 2008). Although hydrolysis of 1a proceeds well with only 1 equiv of LiBr at room temperature, the reaction is quite slow and 2 was obtained in 90% after 40 h (Entry 8). The reaction time was considerably reduced, as expected, when the reaction was carried out under reflux to afford 2 in quantitative yield in only 5.5 h (Compare entries 3 and 9). Screening the reaction varying the equivalents of t-BuNH2, LiBr and reaction time (Entries 10-13) allow us to establish that the excellent reaction conditions for the hydrolysis of 1a into 2, namely in the presence of 10 equiv of t-BuNH2 and 3 equiv of LiBr under reflux, occurred in 2 h (Entry 11).
Since addition of LiBr to the reaction mixture for the hydrolysis of 1a into 2 resulted in better reaction times, we pursued the use of this salt as the catalyst for further hydrolysis. Hydrolysis of esters without LiBr were also carried out at the same time for comparison. Thus, a wide range of structurally varied carboxylic esters including aliphatic, unsaturated, aromatic and an amino ester underwent hydrolysis by this procedure. Excellent yields have been achieved including reactions involving sterically hindered esters. The results are summarized in Tables 1-4. As is shown in Table 1 (Entries 14-23) the steric effect of the alkoxide leaving group influences the efficacy of ester hydrolysis. Under similar conditions methyl ester 1b afforded the product 2 in quantitative yield in 1.75 h (Entry 15), whereas for i-propyl, benzyl and allyl esters 1c, 1e and 1f longer reaction times were needed, 10, 8, and 6 h, respectively (Entries 17, 21 and 23). In the case of tert-butyl ester 1d (Entry 19) only 67% of 2 was observed in the 1H NMR spectrum of the reaction crude even after 92 h.
bCalculated by 1H NMR analysis of the crude material.
As is shown in 1a-f the steric effect of the alkoxy group of the ester influences the rate of ester hydrolysis in the order Me ≈ Et > Bn ≈ allyl > i-Pr >> t-Bu. For the ester hydrolysis of 1a-f in the presence of LiBr, the reaction rates were from 2.5 to 5 fold faster than in those experiments carried out in the absence of the salt.
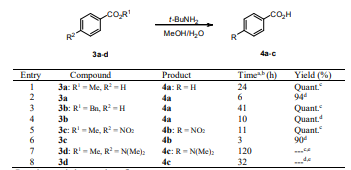
Esters of benzoic acid 3a-d were also hydrolyzed with the above standard reaction conditions and transformations were very clean and gave also very high yields (Table 2). Esters with substituent groups other than electron-donating substituents were hydrolyzed in very high yield (Entries 1-6), while the reaction for compound 3d containing an electron-donating substituent failed even after 32 h (Entries 7 and 8).
A comparison of the results obtained by hydrolysis of esters 1a-f and 3a-d (Tables 1 and 2) led to the conclusion that under this protocol a plausible mechanism could be the nucleophilic attack at the carbonyl ester group by the ˉOH generated in situ by reaction of t-BuNH2 and water. The Li+ should activate the carbonyl ester group to improve base catalyzed O-nucleophilic attack by the ˉOH ion (Firouzabadi, 1999; Saraswathy and Sankararaman 1994; Mojtahedi, 2007).
bReaction carried out with 10 equiv of t-BuNH2.
cReaction carried out without LiBr.
dReaction carried out with 3 equiv of LiBr.
eStarting material was recovered.
The nucleophilicity of t-butylamine has been shown to be considerably less than that of other primary amines of comparable basicity (Minegishi and Kobayashi 2004; Brotzel, 2007; Kumar and Jolly 1999; Kadereit and Waldmann 2001; Fotakopoulou, 2007; Schmidt, 2005; Barbayianni, 2005; Bordusa 2002), undoubtedly because of the steric effect of the t-butyl group. For this reason, nucleophilic attack of the amine on the carbonyl carbon cannot compete with hydrolysis (Table 1, Entries 1-3). The possibility that the amine reacts at the alkyl group of 1 in an SN2 or SN1 manner is ruled out with the observation of the slower reactions of benzyl and allyl esters (Table 1, entries 21 and 23).
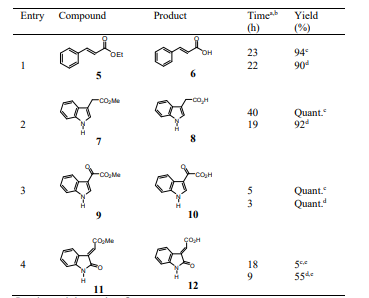
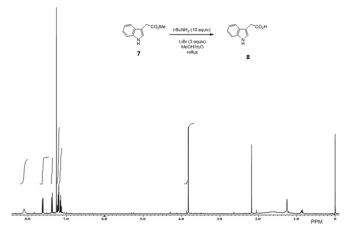
The scope of this reaction was also explored with ester derivatives 5, 7, 9 and 11, which gave the respective carboxylic acids 6, 8, 10 and 12 in high yields as is shown in Table 3. The functional group ketone and alkene survived under these reaction conditions. As a representative example, Figure 2 shows the 1H NMR spectra for the conversion of 7 into 8.
bReaction carried out with 10 equiv of t-BuNH2.
cReaction carried out without LiBr.
dReaction carried out with 3 equiv of LiBr.
eStarting material was recovered.
On the other hand, as the founding of selective deprotection methods of a particular functional group in the presence of others is still one of the most important transformations in organic synthesis, we decided to test the above reaction conditions for the hydrolysis of an ester group in the presence of a labile amino protecting group such as a carbamate. This selective deprotection is very difficult to carried out and only a few methods are reported (Marcantoni, 2001; Yadav, 2002; Chee, 2001; Bartoli, 2000; Wu, 2000; Kaul, 2004).
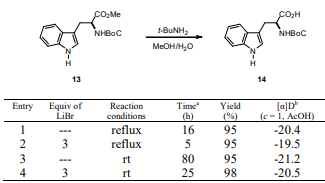
In particular, we examined the deprotection conditions on enantiopure amino ester 13 bearing a methyl ester and a Boc amino protecting group (Table 4). The ester group was selectively hydrolyzed while the carbamate protecting group on the nitrogen remained intact to give the corresponding protected amino acid 14, which was obtained with partial racemization when hydrolysis was carried out in the presence of LiBr (Entry 2). This result provoked a more detailed investigation of the use t-BuNH2/MeOH/H2O with or without LiBr to obtain product 14 without racemization.
bLit.(Marcantoni, 2001) [α]D = -21.1 (c = 1, AcOH).
We found that the reaction of aminoester 13 at room temperature produced the amino-protected acid 14 in quantitative yield without noticeable racemization, although prolonged reaction times were required (Entries 3 and 4).
The evidence that the ester hydrolysis proceeded without racemization under this reaction conditions was provided by comparison of specific rotation of the amino acid 14 with that reported in literature (Marcantoni, 2001).
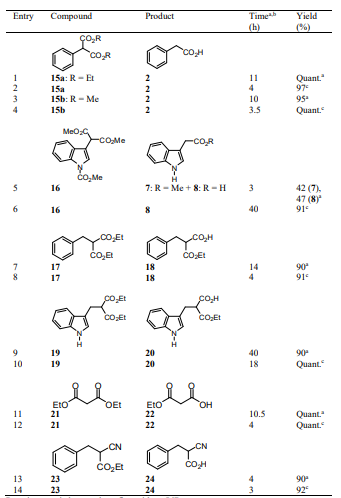
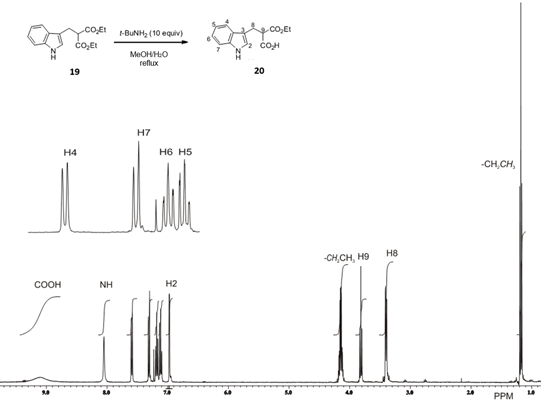
We expanded the scope of this protocol to the hydrolysis of symmetric diesters of malonic acid derivatives 15-17, 19, 21 and 23. When dialkyl phenylmalonates 15a, 15b or 16 were used as substrates, hydrolysis and decarboxylation occurred to afford phenyl acetic acid 2 or 2-indolylacetic acid 8 as the sole products in high yield (Table 5, entries 1-4, 6). However, when we next examined dialkyl malonates 17, 19 and 21 or benzyl cyanoacetate 23 as starting materials, pure half esters 18, 20 and 22 or cyanoacetic acid 24 were obtained in high yields (Entries 7-14), and no decarboxylated products were detected. With these results in hand it is evident that once the half esters of 15 and 16 are formed, their decarboxylation takes place as the negative charge at the alfa position of the respective esters could be stabilized by the aromatic ring, unlike half esters 18, 20, 22 and cyanoacetic acid 24.
bReaction carried out with 10 equiv of t-BuNH2.
cReactions carried out under reflux with 3 equiv of LiBr.
The selectivity in the monohydrolysis of malonate diesters using the methodology developed in this work was evidenced when longer-chain diesteres such as dimethyl succinate and dimethyl glutarate were used since the hydrolysis of these compounds afforded not the corresponding half esters (Niwayama, 2010; Niwayama, 2000; Niwayama, 2007; Rajsfus, 2013; Pitsinos, 2012) but the diacids in high yields.
Although the selectivity for monohydrolysis of malonate esters is not clear, Niwayama has proposed that inter- and/or intramolecular hydrophobic attractive interactions form aggregates in which further hydrolysis could be prevented (Niwayama, 2008).
4. Conclusions
The present procedure using t-BuNH2/MeOH/H2O in combination with LiBr has considerable utility for the carboxylic ester hydrolysis and monohydrolysis of symmetric diesters of malonic acid derivatives. A variety of esters, including aliphatic and aromatic compounds have been subjected to hydrolysis according to this procedure. The uniqueness of this protocol was demonstrated by the ester hydrolysis in the presence of an N-Boc protecting group. The reactions are, in general, very clean and give very high yields. Besides, the operational simplicity, the general applicability, the low cost of the reagents and the high yields enhance its attractiveness and will find useful applications in organic synthesis, particularly for ester deprotection in peptide synthesis.
References
Andrés, G. O.; de Rossi, R. H. (2003) Mechanism of phthalate ester hydrolysis in water and in cyclodextrin mediated reactions. ARKIVOC, x, 127-138.
Andrés, G. O.; Pierini, A. B.; de Rossi, R. H. (2006) Kinetic and Theoretical Studies on the Mechanism of Intramolecular Catalysis in Phenyl Ester Hydrolysis. J. Org. Chem., 71, 7650-7656.
Barbayianni, E.; Fotakopoulou, I.; Schmidt, M.; Constantinou-Kokotou, V.; Bornscheuer, U. T.; Kokotos. G. (2005) Enzymatic Removal of Carboxyl Protecting Groups. 2. Cleavage of the Benzyl and Methyl Moieties. J. Org. Chem., 70, 8730-8733.
Bartoli, G.; Beleggia, R.; Giuli, S.; Giuliani, A.; Marcantoni, E.; Massaccesi, M.; Paoletti, M. (2006) The CeCl.·7H.O–NaI system as promoter in the synthesis of functionalized trisubstituted alkenes via Knoevenagel condensation. Tetrahedron Lett., 6501-6504.
Bartoli, G.; Bellucci, M. C.; Petrini, M.; Marcantoni, E.; Sambri, L.; Torregiani, E. (2000) An Efficient Procedure for the Diastereoselective Dehydration of β-Hydroxy Carbonyl Compounds by CeCl.·7H.O/NaI System. Org. Lett., 2, 1791-1793.
Barton, M. A.; Lemieux, R. U.; Savoie, J. Y. (1973) Solid-phase synthesis of selectively protected peptides for use as building units in the solid-phase synthesis of large molecules. J. Am. Chem. Soc., 95, 4501-4506.
Bhattacharya, S.; Kumari, N. (2009) Metallomicelles as potent catalysts for the ester hydrolysis reactions in water. Coordin. Chem. Rev., 253, 2133-2149.
Billamboz, M.; Bailly, F.; Cotelle, P. (2009) Facile synthesis of 4‐alkoxycarbonylisoquinoline‐1,3‐diones and 5‐alkoxycarbonyl‐2‐benzazepine‐1,3‐diones via a mild alkaline cyclization. J. Heterocyclic Chem., 46, 392-398.
Bordusa, F. (2002) Proteases in Organic Synthesis. Chem. Rev., 102, 4817-4867.
Brotzel, F.; Chu, Y. C.; Mayr, H. (2007) Nucleophilicities of Primary and Secondary Amines in Water. J. Org. Chem., 72, 3679-3688.
Chee, G.-L. (2001) Selective deprotection of isopropyl esters, carbamates and carbonates with aluminum chloride. Synlett, 1593-1595.
Delort, E.; Darbre, T.; Reynmond, J.-L. (2004) A Strong positive dendritic effect in a peptide dendrimer-catalyzed ester hydrolysis reaction. J. Am. Chem. Soc., 126, 15642-15643.
Dhar, D.; Beadham, I.; Chandrasekaran, S. (2003) Proline and benzylpenicillin derivatives grafted into mesoporous MCM-41: Novel organic–inorganic hybrid catalysts for direct aldol reaction. Proc. Indian Acad. Sci., 115, 365-372.
Durow, A. C.; Long, G. C.; O’Connell, S. J.; Willis, C. L. (2006) Total Synthesis of the Chlorinated Marine Natural Product Dysamide B. Org. Lett., 8, 5401-5404.
Fife, T. H.; Singh, R.; Bembi, R. (2002) Intramolecular general base catalyzed ester hydrolysis. The hydrolysis of 2-aminobenzoate esters. J. Org. Chem., 67, 3179-3183.
Firouzabadi, H.; Iranpoor, N.; Karimi, B. (1999) Lithium bromide-catalyzed highly chemoselective and efficient dithioacetalization of α,β-unsaturated and aromatic aldehydes under solvent-free conditions. Synthesis, 58-60.
Fotakopoulou, I.; Barbayianni, E.; Constantinou-Kokotou, V.; Bornscheuer, U.T.; Kokotos, G. (2007) Enzymatic Removal of Carboxyl Protecting Groups. III. Fast Removal of Allyl and Chloroethyl Esters by Bacillus subtilis Esterase (BS2). J. Org. Chem., 72, 782-786.
Fujita, T.; Ogino, K.; Tagaki, W. (1988) Bivalent Copper Ion Complex of a Novel Anionic Surfactant Having Functional Imidazole and Hydroxyl Groups as a Remarkably Active Model of Hydrolytic Metalloenzymes. Chem. Lett., 981-984.
Furlán, R. L. E.; Mata, E. G.; Mascaretti, O. A. (1998) Efficient, non-acidolytic method for the selective cleavage of .-Boc amino acid and peptide phenacyl esters linked to a polystyrene resin. J. Chem. Soc., Perkin Trans. 1, 355-358.
Ghosh, P.; Aubé, J. (2011) Resolution of Carboxylic Acids Using Copper(I)-Promoted Removal of Propargylic Esters under Neutral Conditions. J. Org. Chem., 76, 4168-4172.
Giusti, L. A.; Medeiros, M.; Ferreira, N. L.; Mora, J. R.; Fiedler, H. D. (2014) Polymers containing imidazole groups as nanoreactors for hydrolysis of esters. J. Phys. Org. Chem., 27, 297-302.
Greene, T. W.; Wuts, P. G. M. (1999) Protective Groups in Organic Synthesis, 3rd ed.; John Wiley and Sons: New York.
Guler, M. O.; Stupp, S. I. (2007) A self-assembled nanofiber catalyst for ester hydrolysis. J. Am. Chem. Soc., 129, 12082-12083.
Hu, Y. L.; Jiang, H.; Zhu, J.; Lu, M. (2011) Facile and efficient hydrolysis of organic halides, epoxides, and esters with water catalyzed by ferric sulfate in a PEG1000-DAIL[BF.]/toluene temperature-dependent biphasic system. New J. Chem., 35, 292-298.
Ilankumaran, P.; Verkade, J. G. (1999) P(RNCH.CH.).N: Efficient Catalysts for Transesterifications, Acylations, and Deacylations. J. Org. Chem., 64, 3086-3089.
Iosub, V.; Haberl, A. R.; Leung, J.; Tang, M.; Vembaiyan, K.; Parvez, M.; Back, T. G. (2010) Enantioselective Synthesis of α-Quaternary Amino Acid Derivatives by Sequential Enzymatic Desymmetrization and Curtius Rearrangement of α,α-Disubstituted Malonate Diesters. J. Org. Chem., 75, 1612-1619.
Jarowicki, K.; Kocieński, P. (1999) Protecting groups. J. Chem. Soc., Perkin Trans. 1, 1589-1615.
Jarowicki, K.; Kocieński, P. (2001) Protecting groups. J. Chem. Soc., Perkin Trans. 1, 2109-2135.
Kabalka, G.; Wang, L.; Pagni, R. M. (2001). Potassium fluoride doped alumina: an effective reagent for ester hydrolysis under solvent free conditions. Green Chem., 3, 261-262.
Kadereit, D.; Waldmann, H. (2001) Enzymatic Protecting Group Techniques. Chem. Rev., 101, 3367-3396.
Kaul, R.; Brouillette, Y.; Sajjadi, Z.; Hansford, K. A.; Lubell, W. D. (2004) Selective tert-Butyl Ester Deprotection in the Presence of Acid Labile Protecting Groups with Use of ZnBr.. J. Org. Chem., 69, 6131-6133.
Kimura, E.; Shiota, T.; Koike, T.; Shiro, M.; Kodama, M. (1990) A Zinc(II) Complex of 1,5,9-Triazacyclododecane ([12]aneN.) as a Model for Carbonic Anhydrase. J. Am. Chem. Soc., 112, 5805-5811.
Kirsh J. F.; Jencks, W. P. (1964) Base Catalysis of Imidazole Catalysis of Ester Hydrolysis. J. Am. Chem. Soc., 86, 833-837.
Koshikari, Y.; Sakakura, A.; Ishihara, K. (2012) N,N-Diarylammonium Pyrosulfate as a Highly Effective Reverse Micelle-Type Catalyst for Hydrolysis of Esters. Org. Lett., 14, 3194-3197.
Kumar, I.; Jolly, R. S. (1999) Effect of the α-Methyl Substituent on Chemoselectivity in Esterase-Catalyzed Hydrolysis of .-Acetyl Sulfanylalkanoates. Org. Lett., 1, 207-209.
Lee, S. Y.; Lee, S.; Lee, J.; Lee, H. S.; Chang, J. H. (2013) Biomimetic magnetic nanoparticles for rapid hydrolysis of ester compounds. Mater. Lett., 110, 229-232.
Lesutis, H. P.; Gläser, R.; Liotta, C. L.; Eckert, C. A. (1999) Acid/base-catalyzed ester hydrolysis in near-critical water. Chem. Commun., 2063-2064.
Li, P.; Evans, C. D.; Wu, Y.; Cao, B.; Hamel, E.; Joullié, M. M. (2008) Evolution of the total syntheses of ustiloxin natural products and their analogues. J. Am. Chem. Soc., 130, 2351-2364.
Liotta, D.; Sunay, U.; Santiesteban, H.; Markiewicz, W. (1981) Phenyl Selenide Anion, a Superior Reagent for the SN2 Cleavage of Esters and Lactones. J. Org. Chem., 46, 2605-2610.
Long, W.; Jones, C. W. (2011) Hybrid Sulfonic Acid Catalysts Based on Silica-Supported Poly(Styrene Sulfonic Acid) Brush Materials and Their Application in Ester Hydrolysis. ACS Catal., 1, 674-681.
Ludwig, J.; Bovens, S.; Brauch, C.; Elfringhoff, A. S.; Lehr, M. (2006) Design and Synthesis of 1-Indol-1-yl-propan-2-ones as Inhibitors of Human Cytosolic Phospholipase A.a. J. Med. Chem., 49, 2611-2620.
Mandal, P. K.; McMurray, J. S. (2007). Pd−C-Induced Catalytic Transfer Hydrogenation with Triethylsilane. J. Org. Chem., 72, 6599-6601.
Marcantoni, E.; Massaccesi, M.; Torregiani, E.; Bartolli, G.; Bosco, M; Sambri, L. (2001) Selective Deprotection of .-Boc-Protected tert-Butyl Ester Amino Acids by the CeCl..7 H.O-NaI System in Acetonitrile. J. Org. Chem., 66, 4430-4432.
Mattsson, S.; Dahlström, M.; Karlsson, S. (2007) A mild hydrolysis of esters mediated by lithium salts. Tetrahedron Lett., 48, 2497-2499.
Minegishi, S.; Kobayashi, S.; Mayr, H. (2004) Solvent Nucleophilicity. J. Am. Chem. Soc., 126, 5174-5181.
Mirgorodskaya, A. B.; Yackevich, E. I.; Syakaev, V. V.; Zakharova, L. Y.; Latypov, S. K.; Konovalov, A. I. (2012) Micellization and Catalytic Properties of Cationic Surfactants with Head Groups Functionalized with a Hydroxyalkyl Fragment. J. Chem. Eng. Data, 57, 3153-3163.
Mojtahedi, M. M.; Akbarzadeh, E.; Sharifi, R.; Abaee, M. S. (2007) Lithium Bromide as a Flexible, Mild, and Recyclable Reagent for Solvent-Free Cannizzaro, Tishchenko, and Meerwein−Ponndorf−Verley Reactions. Org. Lett., 9, 2791-2793.
Morwick, T. M. (2006) High-Throughput Ester Hydrolysis with Catch-and-Release Isolation of Carboxylic Acids. J. Comb. Chem., 8, 649-651.
Nakamura, A.; Hamasaki, A.; Goto, S.; Utsunomiya, M.; Tokunaga, M. (2011) Irreversible Catalytic Ester Hydrolysis of Allyl Esters to Give Acids and Aldehydes by Homogeneous Ruthenium and Ruthenium/Palladium Dual Catalyst Systems. Adv. Synth. Catal., 353, 973-984.
Niwayama, S. (2000) Highly Efficient Selective Monohydrolysis of Symmetric Diesters. J. Org. Chem., 65, 5834-5836.
Niwayama, S.; Cho, H. (2009) Practical large scale synthesis of half-esters of malonic acid. Chem. Pharm. Bull., 57, 508-510 and references cited therein.
Niwayama, S.; Cho, H.; Lin, Ch. (2008) Highly efficient selective monohydrolysis of dialkyl malonates and their derivatives. Tetrahedron Lett., 4434-4436 and references cited therein.
Niwayama, S.; Cho, H.; Zabet-Moghaddam, Z.; Whittlesey, B. R. (2010) Remote Exo/Endo Selectivity in Selective Monohydrolysis of Dialkyl Bicyclo[2.2.1]heptane-2,3-dicarboxylate Derivatives. J. Org. Chem., 75, 3775-3780.
Niwayama, S.; Wang, H.; Hiraga, Y.; Clayton, J. C. (2007) Influence of co-solvents in the highly efficient selective monohydrolysis of a symmetric diester. Tetrahedron Lett., 48, 8508-8510.
Parish, E. J.; Miles, D. H. (1973) O-Alkyl cleavage of methyl esters by 1,5-diazabicyclo[5.4.0]undecene-5. J. Org. Chem., 38, 1223-1225.
Pearson, A. J.; Roush, W. R. (1999) Handbook of reagents for organic synthesis. Activating agents and protecting groups. Wiley: New York.
Pitsinos, E. N.; Athinaios, N.; Vidali, V. P. (2012) Enantioselective Total Synthesis of (−)-Laurenditerpenol. Org. Lett., 14, 4666-4669.
Poisson, J.-F.; Orellana, A.; Greene, A. E. (2005) Stereocontrolled Synthesis of (−)-Kainic Acid from trans-4-Hydroxy-l-proline. J. Org. Chem., 70, 10860-10863.
Polyzos, A.; Hughes, A. B.; Christie, J. R. (2007) Catalysis of Aryl Ester Hydrolysis in the Presence of Metallomicelles Containing a Copper(II) Diethylenetriamine Derivative. Langmuir, 23, 1872-1879.
Rajsfus, D. E.; Alter-Zilberfarb, S.; Frimer, A. A. (2013) The synthesis of fluorinated endcaps: Part 1. The effect of C-7 fluorination on the base-catalyzed monohydrolysis of 5-norbornenyl-2,3-diesters. J. Flourine Chem., 148, 49-58.
Salmar, S.; Cravotto, G.; Tuulmests, A.; Hagu, H. (2006) Effect of Ultrasound on the Base-Catalyzed Hydrolysis of 4-Nitrophenyl Acetate in Aqueous Ethanol. J. Phys. Chem. B, 110, 5817-5821.
Salomon, C. J.; Mata, E. G.; Mascaretti, O. A. (1994) Scope and Mechanism of Deprotection of Carboxylic Esters by Bis(tributyltin) Oxide. J. Org. Chem., 59, 7259-7266.
Saraswathy, V. G.; Sankararaman, S. (1994) Chemoselective Protection of Aldehydes as Dithioacetals in Lithium Perchlorate-Diethyl Ether Medium. Evidence for the Formation of Oxocarbenium Ion Intermediate from Acetals. J. Org. Chem., 59, 4665-4670.
Schmidt, M.; Barbayianni, E.; Fotakopoulou, I.; Höhne, M.; Constantinou-Kokotuou, V.; Bornscheuer, U. T.; Kokotos, G. (2005) Enzymatic Removal of Carboxyl Protecting Groups. 1. Cleavage of the tert-Butyl Moiety. J. Org. Chem., 70, 3737-3740.
Scrimin, P.; Tecilla, P.; Tonellato, U. (1992) Cationic Metallovesicles: Catalysis of the Cleavage of /7-Nitrophenyl Picolinate and Control of Copper(II) Permeation. J. Am. Chem. Soc., 114, 5086-5092.
Scrimin, P.; Tecilla, P.; Tonellato, U. (1994) Leaving Group Effect in the Cleavage of Picolinate Esters Catalyzed by Hydroxy-Functionalized Metallomicelles. J. Org. Chem., 59, 18-24.
Seebach, D.; Thaler, A.; Blaser, D.; Ko, S. Y. (1991). Transesterifications with 1,8‐Diazabicyclo[5.4.0]undec‐7‐ene/Lithium Bromide (DBU/LiBr) – Also Applicable to Cleavage of Peptides from Resins in Merrifleld Syntheses. Helv. Chim. Acta, 74, 1102-1118.
Suárez-Castillo, O. R.; Montiel-Ortega, L. A.; Fragoso-Vázquez, M. J.; Meléndez-Rodríguez, M.; Sánchez-Zavala, M. (2008) Transesterifications mediated by .-BuNH.. Tetrahedron Lett., 49, 996-999.
Um, I.-H.; Park, Y.-M.; Fujio, M.; Mishima, M.; Tsuno, Y. (2007) Aminolysis of Y-Substituted Phenyl X-Substituted Cinnamates and Benzoates: Effect of Modification of the Nonleaving Group from Benzoyl to Cinnamoyl. J. Org. Chem., 72, 4816-4821.
Watanabe, T.; Ishikawa, T. (1999) Mild air-oxidation of 1,3-dicarbonyl compounds with cesium salts: Novel α-hydroxylation accompanied by partial hydrolysis of malonate derivatives. Tetrahedron Lett., 40, 7795-7798.
Weijnen, J. G.; Koudijs, A. (1992) Synthesis of Chiral 1,10-Phenanthroline Ligands and the Activity of Metal-Ion Complexes in the Enantioselective Hydrolysis of N-Protected Amino Acid Esters. J. Org. Chem., 57, 7258-7265.
Wu, Y.-q.; Limburg, D. C; Wilkinson, D. E.; Vaal, M. J.; Hamilton, G. S. (2000) A mild deprotection procedure for tert-butyl esters and tert-butyl ethers using ZnBr. in methylene chloride. Tetrahedron Lett., 41, 2847-2849.
Xia, J.; Xu, Y.; Li, S.-a.; Sun, W.-y.; Yu, K.-b.; Tang, W.-x. (2001) Carboxy Ester Hydrolysis Promoted by a Zinc(II) 2-[Bis(2-aminoethyl)amino]ethanol Complex: A New Model for Indirect Activation on the Serine Nucleophile by Zinc(II) in Zinc Enzymes. Inorg. Chem., 40, 2394-2401.
Yadav, J. S.; Subba Reddy, B. V.; Venkateshwara Rao, C.; Chand, P. K.; Prasad, A. R. (2002) A Facile and Selective Cleavage of Prenyl Esters Catalyzed by CeCl .·7 H.O-NaI. Synlett, 137-139.

