



Plant protection
Resistance to leaf rust in cultivars and wheat lines of Paraguay
Resistencia a roya de la hoja en cultivares y líneas de trigo de Paraguay
Resistência à ferrugem da folha em cultivares e linhas de trigo do Paraguai
Agrociencia Uruguay
Universidad de la República, Uruguay
ISSN-e: 2730-5066
Periodicity: Bianual
vol. 27, e997, 2023
Received: 01 February 2022
Accepted: 27 October 2022
Published: 27 March 2023
Corresponding author: ruth.scholz@ipta.gov.py

Abstract: Leaf rust (LR) of bread wheat (Triticum aestvium L.), caused by the fungus Puccinia triticina Eriks, is one of the most important diseases in Paraguay, the Southern Cone and worldwide. The economic importance of the disease is clear considering that two or more fungicide applications are necessary to control it in susceptible cultivars. The best strategy for the management of this disease is through genetic resistance. This research was conducted in Uruguay aiming to postulate the LR resistance genes present in 102 lines and wheat cultivars from Paraguay, and to study their field resistance. The presence of 18 major resistance genes expressed at the seedling stage (Lr1, Lr2, Lr3a, Lr3bg, Lr3ka, Lr9, Lr10, Lr11, Lr16, Lr17, Lr23, Lr24, Lr26, Lr27+Lr31, Lr28, Lr30, Lr42) was postulated based on the reaction to different races of the pathogen. The adult plant resistance gene Lr34 was confirmed in 26% of the materials, based on the molecular marker csLV34. This study also allowed differentiating materials with field resistance that can be explained by the seedling resistance and those with adult plant resistance. Knowledge of the resistance genes present in the germplasm of breeding programs is of paramount importance to establish strategies in order to achieve effective and long-lasting resistance based mainly on the combination of race-non-specific minor genes.
Keywords: leaf rust, resistance genes, adult plant resistance.
Resumen: La roya de la hoja (HR) del trigo pan (Triticum aestvium L.), causada por el hongo Puccinia triticina Eriks, es una de las enfermedades más importantes en Paraguay, en el Cono Sur y a nivel mundial. La importancia económica de la enfermedad es clara cuando se considera que son necesarias dos o más aplicaciones de fungicidas para su control en cultivares susceptibles. La mejor estrategia para el manejo de esta enfermedad es a través de la resistencia genética. Esta investigación fue llevada a cabo en Uruguay con el objetivo de postular los genes de resistencia a HR presentes en 102 líneas y cultivares de trigo de Paraguay y estudiar su resistencia a campo. La presencia de 18 genes mayores de resistencia expresados en plántula (Lr1, Lr2, Lr3a, Lr3bg, Lr3ka, Lr9, Lr10, Lr11, Lr16, Lr17, Lr23, Lr24, Lr26, Lr27+Lr31, Lr28, Lr30, Lr42) fue postulada con base en la reacción frente a distintas razas del patógeno. El gen de resistencia de planta adulta Lr34 fue confirmado en 26% de los materiales con base en el marcador molecular csLV34. Este estudio permitió también diferenciar aquellos materiales cuya resistencia en el campo puede explicarse por su resistencia en plántula y aquellos que poseen resistencia de planta adulta. El conocimiento de los genes de resistencia presentes en el germoplasma de los programas de mejoramiento es de suma importancia para establecer estrategias que logren resistencia efectiva y de larga duración basadas principalmente en la combinación de genes menores de raza no específicos.
Palabras clave: roya de la hoja, genes de resistencia, resistencia de planta adulta.
Resumo: A ferrugem da folha (HR) do trigo-pão (Triticum aestvium L.), causada pelo fungo Puccinia triticina Eriks, é uma das doenças mais importantes no Paraguai, no Cone Sul e no mundo. A importância econômica da doença é clara quando se considera que duas ou mais aplicações de fungicidas são necessárias para o controle da doença em cultivares suscetíveis. A melhor estratégia para o manejo desta doença é através da resistência genética. Esta pesquisa foi realizada no Uruguai, com o objetivo de postular os genes de resistência HR presentes em 102 linhas e cultivares de trigo do Paraguai e estudar sua resistência em campo. A presença de 18 principais genes de resistência expressos em plântulas (Lr1, Lr2, Lr3a, Lr3bg, Lr3ka, Lr9, Lr10, Lr11, Lr16, Lr17, Lr23, Lr24, Lr26, Lr27+Lr31, Lr28, Lr30, Lr42) foi postulada. com base na reação contra diferentes raças do patógeno. O gene de resistência de plantas adultas Lr34 foi postulado em 26% dos materiais com base no marcador molecular csLV34. Este estudo também possibilitou diferenciar aqueles materiais cuja resistência em campo pode ser explicada por sua resistência em plantulas e aqueles que apresentam resistência na planta adulta. O conhecimento dos genes de resistência presentes no germoplasma dos programas de melhoramento é de suma importância para o estabelecimento de estratégias que alcancem resistência efetiva e duradoura baseada principalmente na combinação de genes menores não específicos da raça.
Palavras-chave: ferrugem da folha, genes de resistência, resistência de plantas adultas.
1. Introduction
Leaf rust (LR) of bread wheat (Triticum aestvium L.), caused by the fungus Puccinia triticina Eriks, is one of the most important diseases in Paraguay(1), the Southern Cone(1)(2) and worldwide(3). Reported losses in Argentina from this disease have reached 30% of grain yield(4)(5), and in Brazil and Uruguay they have exceeded 50%(2). In Paraguay, losses of up to 50%(1) have been estimated in Alto Paraná Norte and Canindeyú, where sowing is earlier and epidemics are generally more severe. There are scarce precedents of characterization of the population of P. Triticina in Paraguay. Twenty-three samples collected in Paraguay were studied during 2011, of which the TDT-10,20 and MFP races were most frequently isolated, and other races identified in smaller proportion were MFP-20, MDT-10,20, TDT-10, TFT-10,20, MDP, MFT-10,20, MFP-10,20, MDP-20(6). These races have also been identified in Uruguay, illustrating the race similarity present in Paraguay with the races present in Uruguay. The races present in the countries of the Southern Cone that share the same epidemiological zone to the east of the Andes are generally similar since there are no geographical barriers that prevent the inoculum from moving from one country to another(2).
LR is the main cause of the replacement of commercial cultivars in Paraguay(7) and is also credited as one of the main reasons for the increase in foliar fungicide applications in the crop, which increases production costs(1). It was estimated that more than USD 40 million was spent on fungicide applications in the Southern Cone to control the LR epidemics that occurred on 10 widely planted cultivars during the period 1996-2003(2). However, the main strategy aimed at managing this disease is through genetic resistance(8).
Genetic resistance of wheat to LR is conditioned by a high number of genes. Most of the 74 cataloged LR resistance genes(9) are major genes (LR), expressed from the seedling stage, that produce a hypersensitivity response(10). Resistance based on these genes has been widely used by breeders; however, it is not very durable since initially resistant varieties carrying one or two genes change their behavior and become susceptible when the pathogen develops new virulent races on these genes(11). There are resistance genes that express in adult plants, some of those that produce hypersensitivity (adult plant resistance APR-LR) (Lr12, Lr13, Lr22b, Lr35, Lr37) and have other characteristics of the major genes expressed in seedlings. Other genes that express in adult plants have a minor and additive effect, conditioning quantitative resistance, and non-specific race, and have been the focus of greater interest because they are presumed to condition durable resistance(12). In the field, minor genes determine slow disease development (slow rusting)(13) and do not express high levels of resistance when present alone. However, the combination of 4 or 5 genes confers resistance levels close to immunity(12). This resistance has been called partial resistance(14), adult plant resistance(15), and slow rusting(13); in this article it will be referred to as partial resistance (PR). Four genes from PR to LR have been described, Lr34(16), Lr46(17), Lr67(18) and Lr68(19). An outstanding feature of most of these genes is that they have pleiotropic effects on other pathogens(12)(13)(14)(15)(16)(20)(21)(22). The Lr34 gene, located on chromosome 7DS, was first described in the Frontana cultivar(23).
One of the methodologies used to study genetic resistance conditioned by major genes is the postulation of genes carried out with different races of the pathogen. This method is based on the gene-by-gene concept(24) and has been widely used(22)(25)(26) because it is a fast, low-cost and convenient method for identifying seedling resistance conferred by one or two genes, but may not be appropriate when resistance is more complex(27). It is not possible to use this methodology when races do not have the combination of virulence that determines compatible reaction or susceptibility, nor postulate the presence of PR genes, due to no specific virulence on these genes(22).
The use of molecular markers is another alternative to confirm the presence of disease-resistant genes(28). In the case of wheat LR, there are suitable markers for several major (Lr19, 21, 22a, 25, 29, 32, 39, 47, 50, 51) and minor (Lr34, 46, 67, 68)(29) genes.
The molecular marker csLV34 of the Lr34(30) gene has been used by several researchers in different countries(31)(32)(33)(34)(35).
It is important to know the genes present in the germplasm of a breeding program since it would allow identifying sources of resistance with different genes to be introduced to increase genetic variability, as well as characterizing in greater depth the behavior of commercially used cultivars. This study aims to postulate LR resistance genes present in wheat lines and cultivars in Paraguay.
2. Materials and methods
2.1 Postulation of LR genes expressed at the seedling stage
For the postulation of LR resistance genes, 102 wheat materials of the Wheat Breeding Program of the Wheat Research Program (PIT, Programa de Investigación de Trigo) of Paraguay, some of which are introductions of CIMMYT (Table 1, Supplementary material), were evaluated up to the seedling stage. The Thatcher cultivar (Tc) was included as susceptible control and monogenic differential lines in the background of Tc developed in Canada (Lr1, 2a, 2b,2c, 3, 3bg, 3ka, 9, 10, 11, 14a, 14b, 16, 17, 19, 20, 21, 23, 24, 25, 26, 27+31, 28, 29, 30, 32, 33, 36, 37, 38, 41, 42, 47, 51)(36). These lines were selected since they represent genes commonly present in improved bread wheat germplasm and are the ones that can postulate in Paraguayan materials.
2.2 Races of Puccinia triticina
A selection of 19 races of P. Triticinawas isolated from samples collected in Uruguay, which are preserved in the race bank of the Rust Laboratory of INIA La Estanzuela (Table 1), two for their high frequency in the population of the pathogen in the annual survey of races present in Uruguay during 2012 (MFP and TDT-10,20)(6), and the rest to represent different combinations of avirulence/virulence that allow discriminating the presence of different LR genes expressed in seedlings.
| Racesa | Avirulence / virulence formula |
| CHT | 1,2a,2c,9,24,10,14a,20,21,23,27+31,19,25,ICarpintero,39,42,47,2b,29,32,33,34,35,36,38,44,46,47,51,B,52/3,16,26,3ka,11,17,30,14a,14b,3bg,18,28,12,13,15,22a,22b,37 |
| DBB-10,20 | 1,2a,3,9,16,24,26,3ka,11,17,30,14a,21,27+31,19,3bg,18,25,28,ICarpintero,39,42,47,2b,12,13,15,29,32,35,36,37,38,44,46,47,51,53/2c,10,14b,20,23,22a,22b,33,34,B |
| KDG-10,20 | 9,16,26,3ka,17,30,21,23,19,3bg,25,ICarpintero,39,42,47,29,32,36,37,38,44,46,47,51,B,52/2a,2c,3,24,11,10,14a,14b,20,27+31,18,28,2b,12,13,15,22a,22b,34,35,37,38 |
| LPG-10 | 2a,2c,3,16,26,3ka,17,30,20,23,3bg,27+31,33,13+34,B,21/1,9,24,11,10,14a,14b,18,13,15 |
| MCD-10,20 | 2a,2c,9,16,24,3ka,11,30,21,23,19,25,28,ICarpintero,39,42,47,2b,22b,29,32,33,35,36,44,46,47,51/1,3,26,17,10,14a,14b,20,27+31,3bg,12,13,15,22a,22b,34,37,38,B,52 |
| MCP-10 | 2a,2c,9,16,24,11,20,21,23,19,25,28,ICarpintero,39,42,47,2b,29,32,33,34,36,44,46,47,51/1,3,26,3ka,17,30,10,14a,27+31,3bg,18,12,13,15,22a,22b,35,37,38,B,52 |
| MCR-10 | 2a,2c,9,16,24,17,20,21,19,25,ICarpintero,39,42,47,2b,29,32,33,36,37,38,44,47,51,52/1,3,26,3ka,11,30,10,14a,14b,23,27+31,3bg,28,12,13,15,22a,22b,34,35,46,B |
| MCT-10 | 2a,2c,9,16,24,20,21,23,19,25,28,ICarpintero,39,42,47,2b,29,32,33,34,35,36,44,47,51,53,47,51,52/1,3,26,3ka,11,17,30,10,14a,14b,27+31,3bg,18,12,13,15,22a,22b,37,38,46,B |
| MDT | 2a,2c,9,16,26,10,20,21,23,27+31,19,18,25,28,39,42,47,2b,22b,29,32,33,34,36,47,51,52/1,3,24,3ka,11,17,30,14a,14b,3bg,13,15,22a,22b,35,36,37,38,44,46,B |
| MFP | 2a,2c,9,16,11,10,20,21,27+31,19,18,25,28,39,42,47,2b,29,32,33,34,36,37,47,51,52/1,3,24,26,3ka,17,30,14a,14b,23,3bg,18,ICarpintero,12,13,15,22a,22b,33,34,37,38 |
| MFP-10,20 | 2a,2b,2c,9,16,24,11,21,19,18,25,28,ICarpintero,39,42,47,51,52/1,3,24,26,3ka,17,30,10,14a,14b,20,23,27+31,3bg,12,13,15,22a,22b,34,35,37,38,44,46,B |
| MFP-20 | 2a,2c,9,16,11,10,21,23,27+31,19,25,28,ICarpintero,39,42,47,2b,29,32,33,36,44,47,51,B,52/1,3,24,26,3ka,17,30,14a,14b,20,3bg,18,12,13,15,22a,22b,33,34,35,37,38,46 |
| MFR | 2a,2c,9,16,17,10,20,21,27+31,19,3bg,18,25,ICarpintero,39,42,47,2b,29,32,33,35,37,38,36,44,47,51,B,52/1,3,24,26,3ka,11,30,14a,14b,23,28,12,13,15,22a,22b,34,46 |
| MFT-10,20 | 2a,2c,9,16,18,19,21,25,ICarpintero,39,42,47,2b,29,32,33,34,37,44,46,47,51,52/1,3,24,26,3ka,11,17,30,10,14a,14b,20,23,27+31,3bg,12,13,15,22a,22b,35,36,B |
| MHP-10 | 2a,2c,9,11,18,19,20,21,23,24,25,28,ICarpintero,39,42,47,2b,22b,29,32,33,36,37,38,47,51,52/1,3,16,26,3ka,17,30,10,14a,14b,27+31,3bg,12,13,15,22a,34,35,44,46,B |
| MKD-10 | 2a,2c,3ka,9,11,18,19,20,21,23,25,28,30,ICarpintero,39,42,29,32,33,35,36,38,44,47,51,52/1,3,16,24,26,17,3ka,11,17,10,14a,14b,27+31,3bg,2b,12,13,15,22a,22b,34,37,46,B |
| MMD-10,20 | 2a,2c,3ka,11,16,18,19,21,23,24,25,27+31,28,30,ICarpintero,39,42,47,2B,29,32,33,36,44,47,51,52/1,3,9,26,17,10,14a,14b,20,3bg,12,13,15,22a,22b,34,35,37,38,46,B |
| SPG-10 | 3,3ka,3bg16,17,19,20,21,25,27+31,30,ICarpintero,39,42,47,29,32,33,35,36,44,47,51,B,52/1,2a,2c,9,24,26,11,10,14a,14b,23,18,28,2b,12,13,15,22a,22b,34,37,38,46 |
| TDT-10,20 | 9,16,18,19,21,25,28,ICarpintero,39,42,47,29,32,33,34,37,47,51,52/1,2a,2c,3,24,26,3ka,11,17,30,10,14a,14b,20,23,27+31,3bg,2b,12,13,15,22a,22b,35,36,38 |
a Long and Kolmer 1989. The inclusion of 10 and/or 20 after the denomination of the race, indicates virulence on these genes.
The spores of these races were preserved in vacuum glass tubes in a refrigerator at 5 ºC. To increase the inoculum of the races, 15 seeds of susceptible material Little Club (LC) were sown in a 10-cm-diameter pot with a mixture of soil, vermiculite, sand and substrate (Biofer nursery, Riverfilco; Biofer Ltd., Montevideo) in a 1:1:1:1 ratio. When the plants emerged, each pot was treated with 20 cm. of a maleic hydrazide solution (0.36 g/l) to stop the development of the plants and intensify the spore production.
Each pot of LC was inoculated with spores of a different race, suspended in Soltrol 170 mineral oil (Phillips Petroleum Co., Borger, TX). The pots were placed in a wet chamber (100% relative humidity) for about 16 hours.
Subsequently, the pots were moved to the greenhouse with a temperature of 20-25 ºC and 6 to 8 hours of additional light (Son T 400w of sodium). To prevent cross-contamination, a PVC cage was placed on each pot connected to a tube that released a stream of air. Approximately 2 weeks after inoculation, the inoculum was collected and placed in glass tubes that were vacuum sealed and stored in a refrigerator at 4-6 °C(37).
2.3 Resistance in the seedling stage
For the evaluation of seedlings, 28 materials were planted using 6 to 8 seeds per material, in pots (45.5×28.0×7.7 cm) with the above mentioned substrate. The susceptible control Tc was included in each pot; two repetitions were planted per material and race. The materials were inoculated with the 19 races individually, following the procedure described for the increase of races. At 12 days after inoculation, the type of infection (TI) was evaluated according to the scale described by Stakman and others(38), where TI 0 = immune response, without uredinia or necrosis; TI; (fleck) = necrotic lesions without sporulation; TI 1 = small uredinia surrounded by necrosis; TI 2 = small uredinia surrounded by chlorosis; TI 3 = moderate uredinia without chlorosis or necrosis; TI 4 = large uredinia without chlorosis or necrosis(38). The symbols + and - were used to indicate larger and smaller uredinia compared to typical TI, respectively(3).
The TI patterns of the Paraguayan materials were compared with the TI of the lines carrying unique LR resistance genes, to postulate which resistance genes are present in the materials. To postulate the present genes, the TI of the materials under study was considered equal to or less than the TI produced by the Lr lines.
2.4 Behavior in adult plant (field)
Paraguayan materials (Table 2, Supplementary material) were evaluated under field conditions and natural infection of the pathogen during the autumn-winter of 2012 at the National Institute of Agricultural Research (INIA) La Estanzuela, Semillero locality, department of Colonia (LE: latitude 34.3° S, Longitude 57.7° W, elevation of 70 masl) and in the locality of Young, Río Negro Department (Y: latitude 32.7° S, longitude 57.6° W, elevation 76 masl). The sowing was carried out on July, 12 in Young and on July, 24 in La Estanzuela.
The experimental design used was incomplete randomized blocks with 2 replications. Tc and Avocet were used as susceptible control, repeated 6 times in each replication. The plots were 2 ditches 1 meter long. Susceptible edges (ditches with a mixture of different susceptible materials) were planted perpendicular to the plots to homogeneously increase the natural LR inoculum.
LR severity and reaction were assessed 4 times in La Estanzuela, and 3 times in Young, every 2 weeks, approximately, from the stem elongation. The severity of LR was determined according to the Cobb scale with levels of 1 to 100% severity(39). The reaction of the flag leaf was determined according to the scale proposed by Stakman and others(38): R = small uredinia surrounded by necrosis; MR = moderate uredinia surrounded by necrosis; MS = large uredinia surrounded by chlorosis; S = large uredinia without necrosis or chlorosis; M = mixture of reactions R and S(38). The infection coefficient (IC) was calculated as severity by reaction, using a coefficient for each reaction: R = 0.2, MR = 0.4, MRMS or MSMR = 0.6, MS = 0.8 and S = 1.0. The AUDPC (area under the disease progress curve)(36) of the average IC of the two localities was calculated.
The statistical analysis (ANOVA) of the AUDPC-IC was performed using a mixed linear model in the software R, Development Core Team, 2010, using the following linear model:
Yijklm = Gi + E j +GE(ij)+ Rk(j)+ Bl(jk)+ εijkl
where Y: LR AUDPC-IC values, G: effect of genotype i-th (fixed), E: effect of j-th locality (fixed), GE: interaction between i-th genotype and j-th locality, R: repetition within locality (random), B: incomplete block within locality (random), and ε: experimental error with N (0, σ2ε)
The experimental error was equivalent to the genotype component×environment since the significant differences were considered covering the two environments that represented a general environment. Based on this, the minimum significant difference (P < 0.05) was calculated.
The adult plant reaction of the materials recorded in the field was classified into different categories based on the values of the adjusted means of the AUDPC-IC of the two localities, defined according to the distribution of all materials. The AUDPC (0-375: R, 376-780; MR, 781-1000: MRMS, 1001-1500: MS) is calculated from the estimated percentages of the diseased leaf area recorded at different times of the corresponding evaluations. The criterion for this experiment was used according to the development of the disease, considering as R those that have a null to slow development compared to the MS and S genotypes, where genotypes showed faster disease development.
2.5 Confirmation of the presence of Lr34 based on a molecular marker
The molecular marker cs LV34(30) was used to identify the presence of the PR Lr34 gene. DNA extraction, PCR amplification and determination of alleles in agarose gel electrophoresis were performed according to CIMMYT protocols(40). Parula was used as a positive control for the expected band of the allele associated with the presence of Lr34.
3. Results
In seedling tests, susceptible control Tc presented high TI (3 to 4). It was not possible to postulate the ineffective (Lr14a, Lr14b) and effective genes against all races (Lr19, Lr21, Lr25, Lr29, Lr32, Lr39, Lr51 and Lr52). The rest of the tested genes had high and low TI against different races. The information on the studied materials is presented in 3 tables, according to their reaction pattern against the races: resistant to all races (Table 3), resistant to the most frequent races (Table 4), and susceptible to one or both of the most frequent races (Table 2, Supplementary material).
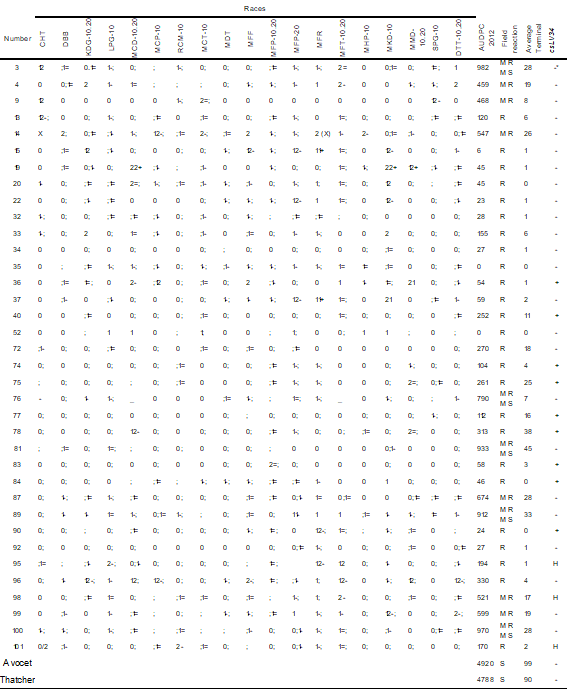
* + presence, - absence of the allele associated with Lr34. H: heterozygous
** + presence of unidentified seedling resistance.
+ presence, - absence of the allele associated with Lr34.H: heterozygous
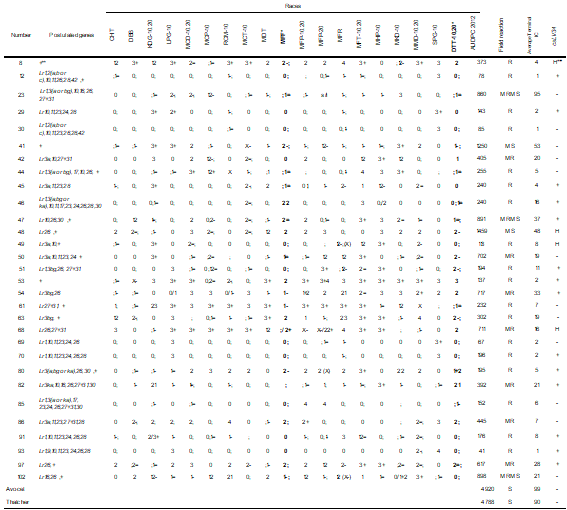
* Prevalent races
** + presence of unidentified seedling resistance.
+ presence, - absence of the allele associated with Lr34. H: heterozygous
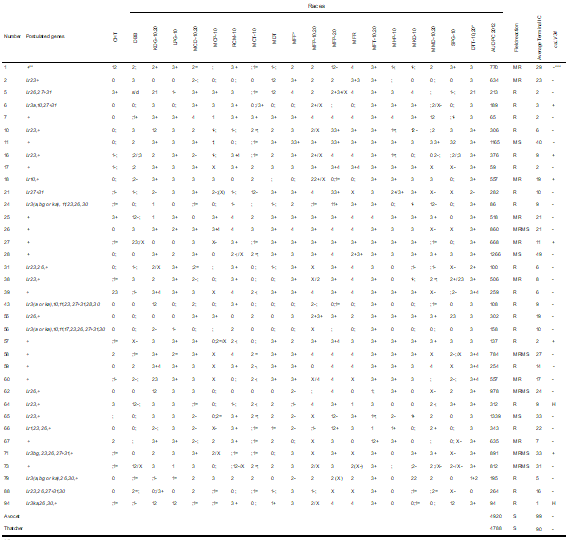
Field infection of susceptible controls Avocet and Tc was high (final infection of 99% and 90%, adjusted mean AUDPC-IC of 4920 and 4788, respectively). The AUDPC ranges of the controls were: Avocet in La Estanzuela (6375-6785), Avocet in Young (3385-4534), Tc in La Estanzuela (5479-6406), and Thatcher in Young (2894-4070). The variance analysis of the AUDPC-IC indicated that there was a significant effect of the materials, environment, and their interaction.
The estimated AUDPC-IC of the materials ranged from 0 to 1459 (Tables 2, 3 and 4) and these values were significantly lower than the estimated AUDPC-IC of the susceptible controls (MDS0.05 1163).
Thirty-six Paraguayan materials were resistant in the seedling stage against the 19 races (Table 3), which did not allow identifying the genes expressed in that stage. Their AUDPC in the field ranged from 0(R) to 982 (MRMS).
With the reaction obtained from 66 Paraguayan materials that were compared with those of lines with unique genes of resistance against races with different combinations of avirulence/virulence (in Table 2, Supplementary material), the presence of 16 genes expressed in seedling was postulated (Lr1, Lr2(a, b or c), Lr3a, Lr3bg, Lr3ka, Lr9, Lr10, Lr11, Lr16, Lr17, Lr23, Lr24, Lr26, Lr27+Lr31, Lr28, Lr30 and Lr42) and additional resistance that could not be identified (Tables 3 and 4).
The number of genes postulated in these materials ranged from 1 to 9. Most materials had different genes or combinations of genes, although some had the same or similar resistance base.
The same genes were postulated in some materials: 69 and 70 (Lr1, Lr10, Lr11, Lr23, Lr24 and Lr26), 5 and 68 (Lr26, 27+31), and 6 and 42 (Lr3a, 10, 27+31), while materials 12 and 30 differed only by the presence of Lr23.
Only one resistance gene was postulated in 12 materials; 6 materials with Lr23; 4 with Lr26, 1 with Lr10, and 1 with Lr3bg; all cases also presented resistance, conferred by LR genes, which was not possible to identify. Two materials presented only the complementary genes Lr27 + Lr31. Another 18 materials possessed seedling strength that could not be identified (+). AUDPC-IC values ranged from 41 to 1459, including materials with field reaction R to MS.
Thirty materials were resistant to the races prevalent during 2012 (MFP and DTT-10, 20, [Table 2, Supplementary material])(6) (Table 4) and showed a range of AUDPC-IC from 41 to 1459. The 36 susceptible seedling materials against MFP and/or TDT-10.20 were R to MS in the field (AUDPC-IC between 59 and 1339, Table 4).
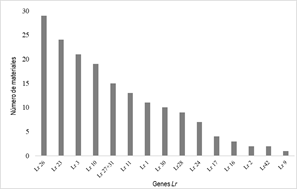
A total of 16 genes were postulated in Paraguayan materials based on their IT against different races. The Lr26 gene was the most frequent, postulated in 29 genotypes. Other genes postulated in more than 15% of the materials were Lr10, Lr3 (jointly considering their 3 alleles) and Lr23. The Lr27+Lr31, Lr11, Lr1 and Lr30 genes are probably present in 10-15% of the materials. Lr28, Lr24, Lr17, Lr3ka, Lr16, Lr2 (a, b or c) and Lr42 were postulated in progressively fewer materials (Figure 1).
Lr34 is probably present in 28% of materials, absent in 63% of materials and 9% was heterozygous for csLV34 (Table 5). Lr34 was postulated in 25% of all-race-resistant materials in the seedling stage, 47% of prevalent race-resistant materials, and 17% of seedling-susceptible materials to one or both prevalent races. Lr34 was postulated in 35% of R materials in the field, 24% of MR materials, 15% of MRMS materials, and was not present in MS materials.
| % of materials | ||||
| Category | +* | -** | H*** | Nº of materials |
| Seedling | ||||
| R to all races | 25.0 | 66.7 | 8.3 | 36 |
| R to prevalent races | 46.7 | 40.0 | 13.3 | 30 |
| S to the two prevalent races | 16.7 | 77.8 | 5.6 | 36 |
| Field | ||||
| R | 34.9 | 55.6 | 9.5 | 63 |
| MR | 23.8 | 66.7 | 9.5 | 21 |
| MRMS | 15.4 | 84.6 | 0.0 | 13 |
| MS | 0.0 | 80.0 | 20.0 | 5 |
| Total | 28.4 | 62.7 | 8.8 | 102 |
*presence, **absence, ***heterozygosity of csLV34
4. Discussion
The postulated genes are alone or in combinations of up to 9 genes per material. It is recognized that the used methodology is not very precise for materials with complex resistances(3). Other studies have postulated up to 5 rust resistance genes in wheat genotypes.
Lr2 has 3 alleles (Lr2a, Lr2b, Lr2c).(41) that could not be differentiated in this study since the used races did not differ in their reaction to them. However, alleles of Lr3 .Lr3a, Lr3bg, Lr3ka)(42)(43) could be differentiated in some cases. Most of the studied materials are introduced lines of CIMMYT or derived from local crossings with lines of this origin, or Brazilian. Therefore, the resistance genes identified in the studied materials come from CIMMYT germplasm and/or regional programs. Eleven of the postulated genes in the studied materials were previously reported in CIMMYT germplasm (Lr1, Lr2a, Lr3a, Lr3bg, Lr10, Lr16, Lr17, Lr23, Lr24, Lr26, Lr27+31)(10)(22)(44)(45)(46)(47)(48). Lr1, Lr3a, Lr10, Lr16, Lr17, Lr24 and Lr26 were reported in cultivars and lines in Argentina, Brazil and Uruguay(25)(32)(33)(49). Lr3bg and Lr23 are present in materials from Brazil and Uruguay(25)(32)(49) and Lr27+Lr31 in materials from Brazil(49). The Lr3ka gene, postulated in 4 genotypes, was first reported in the Argentine cultivar Klein Aniversario(43) and has also been postulated in genotypes of Brazil and Argentina(33)(49). Lr9, postulated in a single material, was transferred from T. umbellulatum to wheat(50). This gene is not widely distributed in germplasm breeding but was reported in some Brazilian and Argentine cultivars(33)(49). Lr11 was described in the Argentine cultivar El Gaucho(51) and in some Brazilian cultivars(49). Lr28 was initially reported in the Australian cultivar Sunland, not very much used in breeding(52), so it is not possible to know how it entered the Paraguayan germplasm. Lr30 was initially described in the Brazilian cultivar Terenzio(53). Lr42 is found in synthetic wheat(54) and is present in modern CIMMYT lines. The seedling resistance that could not be identified probably corresponds to uncatalogued genes, or perhaps the races used do not possess the combinations to postulate other genes, which is common in materials from the region(25), or to races that do not possess the combination for Lr genes that were not tested. However, these genes are relatively important since they are not effective for the races present in the region.
It was not possible to postulate genes in 36 materials that were resistant to all races (Table 3), since, by using this methodology, only the presence of genes or combinations of genes that are ineffective to one or more races can be postulated. Some materials probably possess the resistance genes present in parents. Materials 32 and 34 probably possess Lr42, as they are derived from parents with this gene. Material 33 is derived from a crossing of parents with Lr42 and Lr47 (BABAX/LR42//BABAX*2/3/PAVON 7S3 + LR47) and may have one or both genes. Lr42 was transferred from T. tauschii to wheat(54) and was only ineffective against the race LPG-10. Lr47 was transferred from T. speltoides(55) and was effective for all evaluated races. Resistance in other materials in this group could be conferred by known LR genes or by uncatalogued LR genes, effective to the pathogen population of the region and, therefore, valuable for the LR resistance breeding. To identify the resistance genes present in these materials it is necessary to perform by molecular markers available for Lr19, 21, 22a, 25, 29, 32, 35, 37, 39, 47, 50, 51(29), in order to confirm the presence of these genes and continue introducing them into the genotypes.
According to the consistently high infection and with little variation in susceptible controls Tc and Avocet, a high and uniform LR infection was achieved in the experimental fields, which allowed a good characterization of the resistance of Paraguayan materials in Uruguay. All materials evaluated in the field presented LR infection significantly lower than susceptible controls, which presented a degree of resistance from R to MS. Based on the phenotype, it was not possible to infer the presence of resistance expressed in adult plants, in lines with seedling resistance to all races (Table 3), or resistant to rare races (Table 4), since effective resistance in seedling masks the presence of PR(3). However, Lr34 was postulated in 9 materials through the molecular marker csLV34 (Table 3). Lr34 increases the level of resistance expressed by major genes(56). The presence of Lr34 could reduce future losses if new virulent seedling races were detected on seedling-resistant materials to all races, or if virulent races detected on the seedling resistance of materials resistant to the most prevalent races increased in frequency.
Materials that were susceptible in the seedling stage to the most frequent races (Table 2, Supplementary material) probably possess a resistance in advanced stages of development. Although the experiments were not artificially inoculated, it is assumed that at least the most frequent races identified in the 2012 survey (MFP and TDT-10, 20)(6) were, very probably, present in the experimental fields. These two races are virulent on APR-LR genes, frequent in regional germplasm(2). Probably, these materials’ field resistance is related to the presence of minor genes of additive effect that determines low rusting(13) and confer PR(14). These types of genes do not express high levels of resistance on their own. However, the combination of 4 or 5 PR genes confers resistance levels close to immunity(12)(57)(58). According to this concept, lines under field conditions with MS to R response would have a progressively greater number of PR genes. Silva and others(31) reported the presence of Lr34 and Lr68 PR genes for line 65 (SUZ6/OPATA), which is consistent with the good field behavior of this material(31).
The presence of the Lr34 gene was postulated in 28% of the evaluated materials. The csLV34 marker used to postulate the presence of Lr34 has also been used by other researchers(25)(32)(33)(34)(35). Although it is not a perfect marker (within the gene), it is very close (0.4 cM)(30) and it is considered to have very good diagnostic power(34). Lr34 was first described by Dyck and others(23) in the Brazilian cultivar Frontana(23) and it is present in the germplasm of CIMMYT(12)(13)(20)(22)(47) and the region(32)(33)(34)(35). Its presence in the regional germplasm is also a relevant contribution to the control of wheat diseases since it has conferred durable resistance to LR and has a pleiotropic effect for resistance to stripe rust (Yr18)(22)(52), stem rust (Sr57)(59), powdery mildew (Pm38)(20)(21) and spot blotch (Sb1)(13)(60). As it is associated with the phenotypic character of leaf tip necrosis (LTN1)(22), it can be selected indirectly.
According to the levels of resistance expressed in adult plants, other genes of APR and major genes would also be present, in addition to Lr34, since this gene expresses moderate levels of resistance when present on its own. Diversity in this characteristic is desirable to achieve high and stable PR levels, as well as confirming the presence and/or introgressing other genes that confer this type of resistance(13), such as Lr46, Lr67, Lr68, and other QTLs associated with PR that have not yet been characterized(61). The introgression of Lr68 in Paraguayan germplasm would be especially interesting since this gene has a greater effect than the Lr34 gene in some South American countries(30)(62).
When using major resistance genes, it is essential to know which genes are present in the used germplasm, so as to combine them with genes of different effective resistance, expanding the diversity and duration of its resistance. While the presence of race-specific LR genes is common in cultivars used by farmers and in the germplasm of wheat breeding programs, the use of race-non-specific gene combinations that confer PR is the best alternative to achieve high and durable resistance levels, which decreases the need for chemicals. Being environmentally and economically friendly, it could also allow for better-integrated management.
Before designing the resistance improvement strategy in the Paraguayan Wheat Improvement Program, the resistance of the selected materials in this study should be confirmed, under local conditions, since environmental conditions can affect the expression of PR(62) and the resistance conferred by major genes(63). The pathogen’s diversity can affect the effectiveness of the materials with major gene resistance, according to the virulence of specific genes present. While there is evidence of similarity in the population of P. triticina in the epidemiological zone encompassing Uruguay and Paraguay, the pathogen’s population is highly variable both spatially and temporally, including many races during each growing season(2), which also indicates the need to test the materials locally.
To accelerate the use of this resistance, molecular markers that have been developed for catalogued PR genes can be used to facilitate breeding. This study demonstrates that there are a significant number of materials with PR to LR on which it will be possible to expand diversity and accumulate additional genes, to achieve stable resistance without changes in the behavior of cultivars and in the population of the pathogen. Alternatively, materials with resistance conferred by LR genes effective against all races of the pathogen may be used in combination with PR genes, to avoid high losses in case new virulent races of the pathogen emerge on LR resistance. If LR resistance is used, monitoring the pathogen population to identify new virulent races on the used LR genes is an additional tool that allows taking early measures such as alerts for disease control and replacement of affected varieties.
Acknowledgments
Thanks to INIA, for allowing this research study. To Silvia Germán, for all the scientific support; to Silvia Pereira and Paula Silva for their help, and to all the officials of the rust laboratory of INIA-La Estanzuela. To the Research Program of Paraguay for providing genetic materials and to Dr. Man Mohan Kohli, for his constant support.
References
1. Quintana de Viedma L. Roya de la hoja en trigo. ABC [Internet]. 2009 Aug 11 [cited 2022 Nov 25]. Available from: http://bit.ly/3TZWFQA.
2. Germán S, Barcellos A, Chaves M, Kohli M, Campos P, Viedma L. The situation of common wheat rusts in the Southern Cone of America and perspectives for control. Aust J Agric Res. 2007;58:620-30.
3. Kolmer JA. Genetics of resistance to wheat leaf rust. Annu Rev Phytopathol. 1996;34(1):435-55.
4. Annone JG. Roya de la hoja del trigo. In: Importancia económica y estrategias para reducir los efectos sobre la producción. Córdoba: INTA EEA Marcos Juárez; 2006. p. 26-8. (Informe de actualización técnica; 1).
5. Galich MT. Enfermedades del trigo en el Área Pampeana Norte y su manejo. Córdoba: INTA EEA Marcos Juárez; 1998. 4p. (Información para extensión; Nº 51).
6. Scholz R, Pereyra S, García R, Germán S. Identificación de razas de roya de la hoja del trigo presentes en Uruguay durante 2011-2012. Agrociencia (Uruguay). 2019;23(1):28-36.
7. Kohli MM, Cubilla LE, Cabrera G. Del grano al pan: Tercer Seminario Nacional de Trigo. Asunción: CAPECO; 2010. 168p.
8. Kohli MM, Gérman S. La resistencia genética a enfermedades de trigo en el Cono Sur: panorama pasado, actual y futuro de la roya de la hoja. Paper presented at Jornada Técnica del Manejo integrado de enfermedades en cultivos extensivos; 2003 Sep 16 – 17; Buenos Aires.
9. McIntosh RA, Dubcovsky J, Rogers W, Morris C, Appels R, Xia XC. Catalogue of gene symbols for wheat: 2013–2014 supplement [Internet]. [place unknown: publisher unknown]; 2013 [cited 2022 Dec 1]. 31p. Available from: https://bit.ly/3ue9Mmy.
10. Huerta-Espino J, Singh RP, Villaseñor-Mir HE, Espitia-Rangel E, Leyva-Mir SG. Postulación de Genes de Resistencia a la Roya de la Hoja (Puccinia triticina Ericks.) en Plántula y Planta Adulta en Genotipos Élite de Trigo Harinero (Triticum aestivum). Rev Mex fitopatol. 2003;21(3):239-47.
11. Germán S, Díaz M, Pereyra S. Royas y oídio de trigo y cebada. In: Pereyra S, Díaz M, Germán S, Cabrera K. Manejo de enfermedades en trigo y cebada. Montevideo: INIA; 2011. p. 159-89.
12. Singh RP, Huerta-Espino J, Bhavani S, Herrera-Foessel SA, Singh D, Singh PK, Velu G, Mason RE, Jin Y, Njau P, Crossa J. Race non-specific resistance to rust diseases in CIMMYT spring wheats. Euphytica. 2011;179(1):175-86.
13. Singh RP. Pros and cons of utilizing major, race-specific resistance genes versus partial resistance in breeding rust resistant wheat. In: Borlaug Global Rust Initiative: 2012 Technical Workshop; 2012 Sep 1-4; Beijing, China [Internet]. [place unknown]: BGRI; 2012 [cited 2022 Nov 14]. p. 57-65. Available from: http://bit.ly/3hvabOq.
14. Parlevliet JE. Partial resistance of barley to leaf rust, Puccinia hordei: I. Effect of cultivar and development stage on latente period. Euphytica. 1975;24(1):21-7.
15. Singh RP, Huerta-Espino J. Effect of leaf rust resistance gene Lr34 on components of slow rusting at seven growth stages in wheat. Euphytica. 2003;129(3):371-6.
16. Dyck PL, Kerber ER, Lukow OM. Chromosome location and linkage of a new gene (Lr33) for reaction to Puccinia recondita f. sp. tritici. Genome. 1987;29:463-6.
17. Singh RP, Mujeeb-Kazi A, Huerta-Espino J. Lr46: a gene conferring slow-rusting resistance to leaf rust in wheat. Phytopathology. 1998;88(9):8904.
18. Hiebert CW, Thomas JB, McCallum BD, Humphreys DG, DePauw RM, Hayden MJ, Mago R, Schnippenkoetter W, Spielmeyer W. An introgression on wheat chromosome 4DL in RL6077 (Thatcher*6/PI 250413) confers adult plant resistance to stripe rust and leaf rust (Lr67). Theor Appl Genet. 2010;121:1083-91.
19. Herrera-Foessel SA, Singh RP, Huerta-Espino J, Rosewarne GM, Periyannan SK, Viccars L, Calvo-Salazar V, Lan C, Lagudah ES. Lr68: a new gene conferring slow rusting resistance to leaf rust in wheat. Theor Appl Genet. 2012;124(8):1475-86.
20. Lillemo M, Asalf B, Singh RP, Huerta-Espino J, Chen XM, He ZH, Bjornstad A. The adult plant rust resistance loci Lr34/Yr18 and Lr46/Yr29 are important determinants of partial resistance to powdery mildew in bread wheat line Saar. Theor Appl Genet. 2008;116(8):1155-66.
21. Spielmeyer W, McIntosh RA, Kolmer JA, Lagudah ES. Powdery mildew resistance and Lr34/Yr18 genes for durable resistance to leaf and stripe rust cosegregate at a locus on the short arm of chromosome 7D of wheat. Theor Appl Genet. 2005;111(4):731-5.
22. Singh RP. Genetic association of leaf rust resistance gene Lr34 with adult plant resistance to stripe rust in bread wheat. Phytopathology. 1992;82:835.
23. Dyck PL, Samborski DJ, Anderson RG. Inheritance of adult plant leaf rust resistance derived from the common wheat varieties Exchange and Frontana. Can J Genet Cytol. 1966;8:665-71.
24. Flor HH. The complementary genetic system in flax and flax rust. Adv Genet. 1956;8:29-54
25. Germán SE, Kolmer JA. Leaf Rust Resistance in Selected Uruguayan Common Wheat Cultivars with Early Maturity. Crop Sci. 2012;25:601-8.
26. Loegering WQ, Mcintosh RA, Coleman H, Burton CH. Compute analysis of disease data to derive hypothetical genotypes for reaction of host varieties to pathogens. Can J Genet Cytol. 1971;13(4):742-8.
27. Germán S. Genetics of Leaf Rust Resistance of Selected Uruguayan Wheat Cultivars [doctoral’s thesis]. Manitoba (CA): University of Manitoba; 1996. 135p.
28. Collard BCY, Jahufer MZZ, Brouwer JB, Pang ECK. An introduction to markers, quantitative trait loci (QTL) mapping and marker-assisted selection for crop improvement: the basic concepts. Euphytica. 2005;142:169-96.
29. MAS wheat. Leaf rust resistance genes [Internet]. Davis: University of California; 2021 [cited 2022 Dec 1]. Available from: https://bit.ly/3ufIo7P.
30. Lagudah ES, McFadden H, Singh RP, Huerta-Espino J, Bariana HS, Spielmeyer W. Molecular genetic characterization of the Lr34/Yr18 slow rusting resistance gene region in wheat. Theor Appl Genet. 2006;114(1):21-30.
31. Silva P, Calvo-Salazar V, Condón F, Quincke M, Pritsch C, Gutiérrez L, Castro A, Herrera-Foessel S, von Zitzewitz J, Germán S. Effects and interactions of genes Lr34, Lr68 and Sr2 on wheat leaf rust adult plant resistance in Uruguay. Euphytica. 2015;204(3):599-608.
32. Germán SE, Kolmer JA. Leaf rust resistance in selected late maturity, common wheat cultivars from Uruguay. Euphytica. 2014;195:57-67.
33. Vanzetti LS, Campos P, Demichelis M, Lombardo LA, Aurelia PR, Vaschetto LM, Bainotti CT, Helguera M. Identification of leaf rust resistance genes in selected Argentinean bread wheat cultivars by gene postulation and molecular markers. Electron J Biotechnol. 2011;14(3):1-17.
34. Kolmer JA, Singh RP, Garvin DF, Viccars L, Harinder MW, Huerta-Espino J, Ogbonnaya FC, Raman H, Orford S, Bariana HS, Lagudah ES. analysis of the Lr34/Yr18 rust resistance region in wheat germplasm. Crop Sci. 2008;48(5):1841-52.
35. Singh D, McIntosh RA, Park RF. Characterization of wheat leaf rust resistance gene Lr34 in Australian wheats using components of resistance and the linked molecular marker csLV34. Aust J Agric Res. 2007;58(11):1106-14
36. Long DL, Kolmer JA. A North American System of Nomenclature for Puccinia recondita f. sp. tritici. Phytopathology. 1989;79:525-9.
37. Roelfs AP, Singh RP, Saari EE. Las royas del trigo: conceptos y métodos para el manejo de esas enfermedades. México: CIMMYT; 1992. 81p.
38. Stakman EC, Stewart DM, Loegering WQ. Identification of physiologic races of Puccinia graminis var. tritici. Washington: USDA; 1962. 53p.
39. Peterson RF, Campbell AB, Hannah AE. A diagrammatic scale for estimating rust intensity on leaves and stems of cereals. Can J Res. 1948;26(5):496-500.
40. CIMMYT. Laboratory protocols: CIMMYT applied molecular genetics laboratory. Mexico: CIMMYT; 2005. 5p.
41. Dyck PL, Samborski DJ. Inheritance of virulence in Puccinia recondita on alleles at the Lr2 locus for resistance in Wheat. Can J Genet Cytol. 1974;16:323-32.
42. Browder LE. A compendium of information about named genes for low reaction to Puccinia recondita in wheat. Crop Sci. 1980;20:775-9.
43. Haggag MEA, Dyck PL. The inheritance of leaf rust resistance in four common wheat varieties possessing genes at or near the Lr3. Can J Genet Cytol. 1973;15(1):127-34.
44. Villaseñor-Espín OM, Huerta-Espino J, Leyva-Mir SG, Villaseñor-Mir E, Espitia-Rangel E. Análisis de virulencia de la roya de la hoja (Puccinia triticina Eriks.) del trigo (Triticum aestivum L.) en los Valles Altos de México. Rev Mex fitopatol. 2003;21(1):56-62.
45. Huerta–Espino J, Singh RP. Misconceptions on the durability of some adult leaf rust resistance genes in wheat. In: Proceedings of the 9th European and Mediterranean Cereal Rusts & Powdery Mildews Conference; 1996 Sep; Lunteren, Netherlands. Wageningen: IPO; 1996. p. 109-11.
46. Singh RP. Resistance to Leaf Rust in 26 Mexican Wheat Cultivars. Crop Sci. 1993;33(3):633-7.
47. Singh RP, Rajaram S. Genetics of adult-plant resistance to leaf rust in ‘Frontana’ and three CIMMYT wheats. Genome. 1992;35(1):24-31
48. Singh RP, Rajaram S. Resistance to Puccinia recondita f. sp. tritici in 50 Mexican bread wheat cultivars. Crop Sci. 1991;31(6):1472-9
49. Zoldán SM, Barcellos AL. Postulação de genes (Lr) de resistência a ferrugem da folha em cultivares brasileiras de trigo. Fitopatol bras. 2002;27(5):508-16.
50. Sears ER. The transfer of leaf rust resistance from Aegilops umbellulata into wheat. In: Genetics in Plant Breeding: Report of Symposium Held May 21 to 23, 1956. Upton: Brookhaven National Laboratory; 1956. p. 1-21.
51. Samborski DJ, Dyck PL. Inheritance of virulence in Puccinia recondita on six backcross lines of wheat single genes for resistance to leaf rust. Can J Bot. 1976;54(14):1666-71.
52. McIntosh RA, Wellings CR, Park RF. Wheat rusts: an atlas of resistance genes. Australia: CSIRO; 1995. 178p.
53. Dyck PL, Kerber ER. Aneuploid analysis of a gene for leaf rust resistance derived from the common wheat cultivar Terenzio. Can J Genet Cytol. 1981;23:405-9.
54. Cox TS, Raupp WJ, Gill BS. Leaf rust-resistance genes Lr41, Lr42 and Lr43, transferred from Triticum tauschii to common wheat. Crop Sci. 1994;34:339-43.
55. Dubcovsky J, Lukaszewski AJ, Echaide M, Antonelli EF, Porter DR. Molecular Characterization of Two Triticum speltoides Interstitial Translocations Carrying Leaf Rust and Greenbug Resistance Genes. Crop Sci. 1998;38:1655-60.
56. Germán SE, Kolmer JA. Effect of gene Lr34 in the enhancement of resistance to leaf rust of wheat. Theor Appl Genet. 1992;84(1):97-105.
57. Herrera-Foessel SA, Singh RP, Lillemo M, Huerta-Espino J, Bhavani S, Singh S, Lan C, Calvo-Salazar V, Lagudah ES. Lr67/Yr46 confers adult plant resistance to stem rust and powdery mildew in wheat. Theor Appl Genet. 2014;127(4):781-9.
58. Navabi A, Tewari JP, Singh RP. Inheritance and QTL analysis of durable resistance to stripe and leaf rusts in an Australian cultivar, Triticum aestivum ‘Cook’. Genome. 2005;48(1):97-107.
59. Singh RP, Herrera-Foessel SA, Huerta-Espino J. Lr34/Yr18/Sr57/Pm38/Bdv1/Ltn1 confers slow rusting, adult plant resistance to Puccinia graminis tritici. In: Disease risk and Food Security. 13th Cereal Rust and Powdery Mildew Conference; 2012 Aug 28 - Sep 1. Beijin: China Agricultural Science and Technology Publishing; 2012. p. 173.
60. Lillemo M, Joshi AK, Prasad R, Chand R, Singh RP. QTL for spot blotch in bread wheat line Saar co-locate to the biotrophic disease resistance loci Lr34 and Lr46. Theor Appl Genet. 2013;126(3):711-9.
61. Li Z, Lan C, He Z, Singh RP, Rosewarne GM, Chen X, Xia X. Overview and Application of QTL for Adult Plant Resistance to Leaf Rust and Powdery Mildew in Wheat. Crop Sci. 2014;54(5):1907-25.
62. Lillemo M, Singh RP, William M, Herrera-Foessel S, Huerta-Espino J, Germán S, Campos P, Chaves M, Madariaga R, Xia XC, Liang SS, Liu D, Li ZF, Lagudah ES. Multiple rust resistance and gene additivity in wheat: lessons from multi-location case studies in the cultivars Parula and Saar. In: Borlaug Global Rust Initiative: 2011 Technical Workshop [Internet]. [place unknown]: BGRI; 2011 [cited 2022 Nov 14]. p. 111-20. Available from: http://bit.ly/3hvgtO2.
63. Dyck PL, Johnson R. Temperature sensitivity of genes for resistance in wheat to Puccinia recondita. Can J Plant Pathol.1983;5:22934.
Supplementary material
| 1 | ITAPÚA40 | BOW ́S ́/VEE ́S ́ | CIMMYT | |
| 2 | ITAPÚA70 | RAYON//VEE#6/TRAP1 | CIMMYT | |
| 3 | ITAPÚA75 | VEE"S"/RL6010/JUP73/3/PRINIA | CIMMYT | |
| 4 | Caninde1 | MILAN/MUNIA | CIMMYT | |
| 5 | Caninde11 | WEEBILL2 | CIMMYT | |
| 6 | Y-06068 | WBLL4//BABAX.1B.1B*2/PRL/3/PASTOR | CIMMYT | |
| 7 | Y-06070 | WBLL1*2/TUKURU | CIMMYT | |
| 8 | E-06247 | BABAX/PASTOR/3/KAUZ*2/YACO//KAUZ | CIMMYT | |
| 9 | E-07056 | ITAPÚA 40/KURUKU | PIT | |
| 10 | Q08057 | ITAPÚA 40/KURUKU | PIT | |
| 11 | Q08071 | ITAPÚA 40/KURUKU | PIT | |
| 12 | E-07094 | ITAPÚA 40/CARCOVE//JUP*5/AMIGO | PIT | |
| 13 | Q08158 | ITAPÚA 40/IAN 10 | PIT | |
| 14 | Q08356 | ITAPÚA-50/ITAPÚA-40 | PIT | |
| 15 | Y-08008 | PFAU/WEAVER*2//TRANSFER#12,P88.272.2 | CIMMYT | |
| 16 | Y-06069 | WBLL4/KASO2//PASTOR | CIMMYT | |
| 17 | Y-06074 | WBLL1*2/TUKURU | CIMMYT | |
| 18 | Y-07096 | CS/TH. SC//3*PVN/3/MIRLO/BUC/4/MILAN/5/TILHI | CIMMYT | |
| 19 | Y-07090 | VEE/MJI//2*TUI/3/PASTOR/4/BERKUT | CIMMYT | |
| 20 | 2527 | PRL/SARA//TSI/VEE#5/3/FINSI | CIMMYT | |
| 21 | Y-09035 | PFAU/WEAVER*2//TRANSFER#12,P88.272.2 | CIMMYT | |
| 22 | E-09246 | E 92225/FCEP 30 | PIT | |
| 23 | Y-08027 | PRINIA/STAR// P SUPERI0R/CRDN | CIMMYT | |
| 24 | Y-09006 | EMB16/CBRD//CBRD | PIT | |
| 25 | Y-09003 | GUS/3/PRL/SARA//TSI/VEE#5/4/FRET2 | CIMMYT | |
| 26 | CD 150 | CD 150 | PIT | |
| 27 | E-08069 | ITAPÚA 40/KURUKU | PIT | |
| 28 | E-08031 | ITAPÚA 45/CORDILLERA 4 | PIT | |
| 29 | E-08032 | ITAPÚA 45/CORDILLERA 4 | PIT | |
| 30 | Y08202 | ND643/2*WBLL1 | CIMMYT | |
| 31 | Y-08006 | BABAX/LR42//BABAX*2/4/SNI/TRAP#1/3/KAUZ*2/TRAP//KAUZ | CIMMYT | |
| 32 | Y09096 | BABAX/LR42//BABAX*2/4/SNI/TRAP#1/3/KAUZ*2/TRAP//KAUZ | CIMMYT | |
| 33 | Y09038 | BABAX/LR42//BABAX*2/3/PAVON 7S3, +LR47 | CIMMYT | |
| 34 | Y09023 | BABAX/LR42//BABAX*2/3/VIVITSI | CIMMYT | |
| 35 | Y-07088 | VEE/MJI//2*TUI/3/PASTOR/4/BERKUT | CIMMYT | |
| 36 | Y-08086 | PARULA/IAN 10 | PIT | |
| 37 | Y-09033 | WBLL1*2/RAMBLING | CIMMYT | |
| 38 | Y-09003 | GUS/3/PRL/SARA//TSI/VEE#5/4/FRET2 | CIMMYT | |
| 39 | Y-09004 | T.DICOCCON PI94625/AE. SQUARROSA (372)//3*PASTOR | PIT | |
| 40 | E-08032 | ITAPÚA 45/CORDILLERA 4 | PIT | |
| 41 | AND 10132 | IAN 10/CANINDE 3 | PIT | |
| 42 | AND 10289 | ITAPÚA 45/CANINDÉ 1 | PIT | |
| 43 | AND 10293 | EMBRAPA 120/WEEBILL2 | PIT | |
| 44 | AND 10139 | IAN 10/CANINDE 3 | PIT | |
| 45 | AND 10144 | IAN 10/ITAPÚA 70 | PIT | |
| 46 | AND 10145 | IAN 10/ITAPÚA 70 | PIT | |
| 47 | AND 10228 | CEP 99173/PEWIT1 | PIT | |
| 48 | AND 10236 | PRL/VEE#6//CLMS/3/ITAPÚA 55 | PIT | |
| 49 | AND 10239 | PRL/VEE#6//CLMS/3/ITAPÚA 45 | PIT | |
| 50 | AND 10376 | KAUZ/MILAN/3/CROC_1/AE. SQUARROSA (205)//KAUZ | CIMMYT | |
| 51 | AND 10378 | SITE/FINSI | CIMMYT | |
| 52 | AND 10379 | KAUZ*2//K134(60)/VEE/3/ATTILA/4/MILAN/KAUZ | CIMMYT | |
| 53 | AND 10381 | KAUZ*2//K134(60)/VEE/3/ATTILA/4/MILAN/KAUZ | CIMMYT | |
| 54 | AND 10383 | MILAN/KAUZ//PASTOR/3/PASTOR | CIMMYT | |
| 55 | AND 10388 | SUZ6/OPATA | CIMMYT | |
| 56 | AND 10482 | KAMBARA2//MILAN/AMSEL | PIT | |
| 57 | AND 10416 | WBLL2//MILAN/AMSEL | CIMMYT | |
| 58 | AND 10479 | KAMBARA2/4/BABAX/LR42//BABAX/3/BABAX/LR42 | PIT | |
| 59 | AND 10491 | CD114//WBLL1*2/TUKURU | PIT | |
| 60 | AND 10483 | CD108/3/SRMA/TUI//BABAX | PIT | |
| 61 | AND 10425 | ITAPÚA 40//RL 6000=Thatcher*7/Webster=Lr2a | PIT | |
| 62 | AND 10499 | TNMU/6/CEP80111/CEP8165/5M/7/WBLL1*2/TUKURU | CIMMYT | |
| 63 | AND 10456 | WBLL4/5/BABAX.1B.1B*2/PRL/3/PASTOR/4/OC 946/PF906 | PIT | |
| 64 | AND 10417 | WBLL2/BR18 | PIT | |
| 65 | AND 10209 | PARAGUA CIAT//MILAN/MUNIA | PIT | |
| 66 | E-07095 | ITAPÚA 40/CARCOVE//JUP*5/AMIGO | PIT | |
| 67 | Q08028 | ITAPÚA 45/CORDILLERA 4 | PIT | |
| 68 | E-07057 | ITAPÚA 40/KURUKU | PIT | |
| 69 | Q08078 | ITAPÚA 40/KURUKU | PIT | |
| 70 | LAJ5018 | PGO//CROC1/AE SQ (224) /3/2*BORL95/4/BAV92/5/PASTOR | CIMMYT | |
| 71 | E-09091 | ITAPÚA 40/CEP 36 | PIT | |
| 72 | E-09244 | E 92225/FCEP 30 | PIT | |
| 73 | E-09256 | E 97034/ORL 99220 | PIT | |
| 74 | E-09252 | E 97034/ENT 980204 | PIT | |
| 75 | E-09200 | IAN 10/ORL 99393 | PIT | |
| 76 | E-09108 | ITAPÚA 40/ E-96001 | PIT | |
| 77 | E-09022 | ITAPÚA 45/ITAPÚA 40 | PIT | |
| 78 | E-09367 | YANGMAI 5*2/4/MOR/VEE#5//DUCULA/3/DUCULA | CIMMYT | |
| 79 | E-09307 | CORDILLERA 4/ORL AL | PIT | |
| 80 | E-09246 | E 92225/FCEP 30 | PIT | |
| 81 | E-09064 | ITAPÚA 40/PROINTAGRANAR | PIT | |
| 82 | E-09404 | BABAX/LR42//BABAX*2/3/TUKURU | CIMMYT | |
| 83 | E-09393 | PARULA/IAN 10 | PIT | |
| 84 | E-09415 | ITAPÚA 60/CANINDE 1 | PIT | |
| 85 | E-09382 | ITAPÚA 40/PEWIT1 | PIT | |
| 86 | E-09383 | CORDILLERA 3/WEEBIL2 | CIMMYT | |
| 87 | E-09384 | CORDILLERA 3/PEWIT3 | CIMMYT | |
| 88 | E-09408 | ITAPÚA 55//P-SUPERIOR/CRDN | PIT | |
| 89 | E-09408 | MILVUS1/ITAPÚA 60 | PIT | |
| 90 | E-09893 | E 97034/ORL 99220 | PIT | |
| 91 | E-10098 | ITAPÚA 75/WEEBILL 2 | PIT | |
| 92 | E-10101 | ITAPÚA 75//PF 953048/IAPAR 18 | PIT | |
| 93 | E-10104 | PARULA/CD 104 | PIT | |
| 94 | E-10105 | PARULA/CD 104 | PIT | |
| 95 | E-10106 | PARULA/CD 104 | PIT | |
| 96 | E-10121 | ITAPÚA 75/Cord 3 | PIT | |
| 97 | E-10126 | ITAPÚA 75/3/ITP35/PF84432//CORD4 | PIT | |
| 98 | E-10132 | E- 2044/Cord 3 | PIT | |
| 99 | E-09760 | ITAPÚA 55//HUW234+LR34*2/PASTOR | PIT | |
| 100 | E-09676 | ITAPÚA 45//TUI/RL 4137 | PIT | |
| 101 | E-09841 | ITAPÚA 60/PROINTAGRANAR | PIT | |
| 102 | E-09628 | ITAPÚA 40/CEP 36 | PIT | |
| Susceptible control | Avocet | |||
| Susceptible control | Thatcher | |||
| Genes | CHT | DBB | KDG-10.20 | LPG-10 | MCD-10.20 | MCP-10 | RCM-10 | MCT-10 | MDT | MFF | MFP-10.20 | MFP-20 | MFR | MFT-10.20 | MHP-10 | MKD-10 | MMD-10.20 | SPG-10 | DTT-10,20 |
| Lr 1 | 0 | 0 | 0 | 4 | 3+ | 3+ | 3+ | 3+ | 3+ | 3+ | 3+ | 3+ | 3+ | 3+ | 3+ | 3+ | 3+ | 3+ | 3+ |
| Lr 2a | 0; | 1- | 3+ | 0 | 0 | 0 | 0; | 0 | 0; | 0; | 0 | 0; | 0 | 0; | 0; | 0 | 0 | 3+ | 3 |
| Lr 2c | 12- | 3+ | 3+ | 0;1= | 0; | 1- | 1- | 1-; | 1-; | 1- | 2=; | 1-; | 0 | 0; | 1-; | 1=; | 0; | 3+ | 3 |
| Lr 3a | 3+ | 0 | 3+ | 0;1= | 3+ | 3+ | 3+ | 3+ | 3+ | 3+ | 3+ | 3+ | 3+ | 3+ | 3+ | 3+ | 3+ | 0; | 3+ |
| Lr 3bg | 3+ | 0 | 12- | 0; | 33+ | 3+ | 2+3 | 2+3 | 3+ | 3+ | 3+ | 23 | 22+ | 3+ | 2+3+ | 33+ | 3+ | 0 | 3+ |
| Lr 3ka | 3+ | 0 | 2=; | 2 | 2= | 3+ | 3+ | 3+ | 3+ | 3+ | 3+ | 23 | 3+ | 3+ | 3+ | 2=; | 0; | 2-; | 3+ |
| Lr 9 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0; | 0; | 0; | 0 | 0 | 0 | 0 | 0 | 3+ | 3+ | 0; |
| Lr 10 | 0; | 3+ | 33+ | 4 | 3+ | 3 | 3+ | 3+ | 2=; | 0; | 3+ | 2=; | 0; | 3+ | 3+ | 3+ | 3 | 4 | 3+ |
| Lr 11 | 3+ | 0 | 3+ | 3+4 | 2 | 2- | 3+ | 3+ | 3+ | 2 | 2= | 2= | 3+ | 3+ | 2=; | 2= | 1-; | 3+ | 3+ |
| Lr 16 | 2+3+ | 12 | 1- | 1= | 1- | 1- | 1-; | 1- | 1-; | 1- | 1- | 1- | 2=; | 1- | 12 | 33+ | 2 | 1- | 1- |
| Lr 17 | 3+ | 0 | 0; | ;1= | 33+ | 3+ | ; | 3+ | 3 | 3 | 3+ | 23+ | 2= | 3+ | 3+ | 3+ | 3+ | ;1= | 3+ |
| Lr 18 | 3+ | 0 | 33+ | 4 | 3 | 3+ | 3+ | 2+3 | 2 | 3 | 2=; | 23 | 2 | 2=; | 2- | 2 | 2= | 3+ | 2= |
| Lr 23 | 2- | 33+ | 2- | 2+ | 2 | 2 | 3+ | 1-; | 2- | 3 | 3+ | 2 | 3 | 3 | 1- | 12- | 2 | 3+ | 3+ |
| Lr 24 | 0; | 0 | 3 | 4 | 0; | 0 | 0; | 0 | 23 | 3+ | 3+ | 3+ | 3+ | 3+ | 0; | 33+ | 0; | 3+ | 3+ |
| Lr 26 | 3+ | 0 | 2 | 2+ | 3 | 3 | 3+ | 3+ | 2 | 3 | 3+ | 23 | 3+ | 3+ | 3+ | 33+ | 2+3 | 3+ | 3+ |
| Lk 27+31 | ; | 0 | 3+ | 4 | 3+ | 33+ | 3 | 3+ | ; | ;1- | 3+ | ;1= | 0; | 3+ | 3+ | 3+ | 2=; | 1-; | 3+ |
| Lr 30 | 3+ | 0; | 2- | 2 | 2= | 3+ | 3+ | 3+ | 3+ | 3 | 3+ | 23 | 3+ | 3+ | 3+ | 2=; | 0 | 2-; | 3+ |
Author notes
ruth.scholz@ipta.gov.py
Additional information
Editors: The following editor approved this article: Carlos Pérez (https://orcid.org/0000-0002-5501-064X)
Universidad de la República, Facultad de Agronomía, Paysandú, Uruguay
Author contribution statement: All authors collaborated, analyzed
and wrote the manuscript. The final version of the manuscript was accepted by
all authors.
Alternative link
https://agrocienciauruguay.uy/index.php/agrociencia/article/view/997/1355 (pdf)

