



Molecular identification of Trypanosoma cruzi in a naturally infected dog from Nicaragua
Identificación molecular de Trypanosoma cruzi en un perro infectado naturalmente de Nicaragua
Revista Iberoamericana de Bioeconomía y Cambio Climático
Universidad Nacional Autónoma de Nicaragua, León, Nicaragua
ISSN-e: 2410-7980
Periodicity: Semestral
vol. 8, no. 15, 2022
Received: 17 November 2021
Accepted: 08 April 2022
Corresponding author: byronfloressomarriba@gmail.com
Resumen: En América Latina, la enfermedad de Chagas es una amenaza importante para la salud pública y los caninos juegan un papel importante en los ciclos de transmisión doméstica de Trypanosoma cruzi. Este reporte presenta un caso de Chagas en un perro mestizo macho de dos meses de edad que fue rescatado y trasladado a la Clínica Veterinaria Privada Royal Pets en la Ciudad de Managua - Nicaragua. El animal se encontraba en estado caquéctico, débil y completamente mojado, presentando temperatura rectal de 33.6 ° C, deshidratación (9%), mucosas pálidas, ganglios linfáticos no reactivos, abdomen distendido sin dolor a la palpación, lesión ulcerosa sanguinolenta en la caja torácica izquierda. En el frotis periférico se detectó el parásito de la sangre Trypanosoma; además, en el análisis de PCR se obtuvo amplificación para Trypanosoma cruzi, pero negativa para Trypanosoma vivax y Trypanosoma evansi. La detección e identificación de este caso podría generar conciencia en el país sobre la importancia de notificar las infecciones caninas como parte de los programas de vigilancia epidemiológica para controlar los casos humanos de la enfermedad de Chagas.
Palabras clave: Trypanosoma cruzi, canino, Nicaragua, PCR, Zoonosis.
Abstract: In Latin America, Chagas disease is a significant public health threat and canines play an important role in the domestic transmission cycles of Trypanosoma cruzi. This report presents a case of Chagas in a two-month-old male mongrel dog that was rescued and taken to the private Royal Pets Veterinary Clinic in the City of Managua - Nicaragua. The animal was in a cachectic state, weak and completely wet, presenting a rectal temperature of 33.6 ° C, dehydration (9%), pale mucous membranes, non-reactive lymph nodes, distended abdomen without pain on palpation, bloody ulcerative lesion in the left rib cage. In peripheral smear examination, the blood parasite Trypanosoma was detected; in addition, in the PCR analysis, amplification was obtained for Trypanosoma cruzi, but negative for Trypanosoma vivax and Trypanosoma evansi. The detection and identification of this case could raise awareness in the country about the importance of reporting canine infections as part of epidemiological surveillance programs to control human cases of Chagas disease.
Keywords: Trypanosoma cruzi, Canine, Nicaragua, PCR, Zoonosis.
Introduction
Chagas disease is an anthropozoonosis caused by Trypanosoma cruzi, and considered as an important reason of the high burden of infectious diseases in Latin America (WHO, 2015). The insect species Rhodnius prolixus, Rhodnius pallescens and Triatoma dimidiata are the vectors responsible for most of the current cases of Chagas disease in the Central American region (Peterson et al., 2019). Dogs play an important role in the domestic transmission cycles of T. cruzi and are considered to be a risk factor for human infection (Vasconcelos et al., 2021), as they are important reservoirs of infection and can potentially act as sentinels for human infections (Roegner et al., 2019).
Chagas disease in humans is characterized by a brief, generally asymptomatic, acute phase followed by a lifelong chronic phase often associated with cardiac and digestive damage (Vasconcelos et al., 2021). Dogs get frequently affected by Chagas disease and develop clinical signs of an acute phase as well as chronic cardiac manifestations similar to those detected in humans (Lizundia et al., 2014; Montenegro et al., 2002). In Nicaragua in 2005, the prevalence of T. cruzi infection in humans was estimated 1.1%, with 25% of the population being at risk of acquiring the infection. Seroprevalence in clinical studies has ranged from 13.1% in an endemic region of the country to 3.2-4.3% in areas with infection control activities (Roegner et al., 2019). Despite this, in Nicaragua there are few studies aimed at addressing to detect infection in canines and these few studies only perform serological tests. Also, veterinarians are not yet familiar with the clinic of the disease in dogs. Therefore, most cases are detected in the peripheral blood smear that is performed in order to search for other more common agents such as Anaplasma spp or Ehrlichia spp., however, when detected in the blood, species identification is not carried out. In recent years, dogs suspected of Chagas disease or with heart failure of unknown origin have been treated at the Royal Pets Veterinary Clinic; it might be posible that contagious infections in humans derived from cycles of zoonotic transmission may be occurring more frequently than currently documented and therefore better surveillance should help define the risk of parasite transmission to humans (Elmayan et al., 2019).This study describes the first molecular detection of Trypanosoma cruzi in a naturally infected dog from Nicaragua.
The following report was based on a case study in a two-month-old male mongrel canine that was rescued. The dog was taken to the private Royal Pets Veterinary Clinic in the City of Managua - Nicaragua, on November 11, 2020 at 9:35 am, in a cachectic state, weak and completely wet, presenting a rectal temperature of 33.6 ° C, dehydration (9%), pale mucous membranes, non-reactive lymph nodes, distended abdomen without pain on palpation, bloody ulcerative lesion in the left rib cage and abundant fleas and ticks (Figure 1). A blood sample was taken from the right cephalic vein, with a 3 ml syringe and a 23G x 1” needle, then placed in a 1 ml purple stopper tube (EDTA) for complete blood count in the laboratory of the Royal Pets Veterinary Clinic using the standard procedures previously described (Sirois, 2018). Additionally, in order to show the presence of hemoparasites, microscopic observation of peripheral blood smear stained with Diff Quick was carried out. A general stool examination was also performed with the following three techniques: 1) simple flotation with hypertonic solution, 2) direct smear with 0.9% saline solution and 3) fecal cytology stained with Diff Quick.
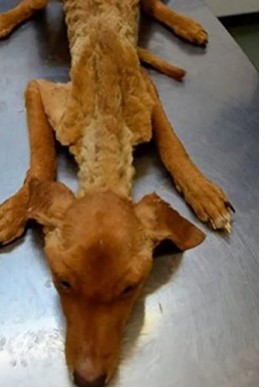
One ml of blood was sent to the molecular diagnostic laboratory, at the Centro Veterinario de Diagnóstico e Investigación (CEVEDI) de la Universidad Nacional Autónoma de Nicaragua, León (UNAN – León), where a polymerase chain reaction (PCR) was performed to identify species of trypanosomes. For DNA extraction, 20 µl of whole blood was taken, using the protocol described in the Qiagen Blood and Tissue DNA kits (Qiagen, Germany). For the identification of the species, the primers described in Table 1 were used. The reaction was carried out in a total volume of 50 µl, containing 25 µl of Master Mix 2X (Promega, USA), 2 µl of each primer (10,000 nM), 16 µL of Nuclease-free water and 5 µl of DNA from the sample. PCR thermal cycling steps for T. vivax and T. evansi were performed according to (Birhanu et al., 2015),, whereas those for the identification of T. cruzi according to (Moser et al., 1989).
| Table 1. Primers used for the identification of the Trypanosome species in the polymerase chain reaction (PCR) |
| Species Forward (5´-3´) Reverse (5´-3´) Product (bp) Reference Trypanosoma vivax CGCAAGTGGACCGTTCGCCT ACGCGGGGCGAACAGAAGTG 239 (Birhanu et al., 2015; Fikru et al., 2014) Trypanosoma cruzi CGAGCTCTTGCCCACACGGGTGCT CCTCCAAGCAGCGGATAGTTCAGG 188 (Moser et al., 1989) Trypanosoma evansi ACAGTCCGAGAGATAGAG ACAGTCCGAGAGATAGAG 436 (Birhanu et al., 2015) |
| bp: base pair |
As a main result, the complete blood count indicates regenerative anaemia (reticulocytes 4.9%) with the specific erythrocyte morphology of a microcytic and hypochromic anaemia, as well as target cells, low levels of haemoglobin, eosinophilia and normoproteinemia (7.2 g /dl) were also found. The rest of the parameters were within the normal range.
In the stool analysis, gastrointestinal parasites were found such as Toxocara canis (17 epg), Ancylostoma (38 epg), Isospora spp (30 epg); in fecal cytology, microcolonies of cocci and diplococci were observed, and incidental yeast infection (> 10 cells per field)
In blood smears trypomastigotes were observed morphologically compatible with Trypanosoma spp (Figure 2). PCR revealed amplification of a 188 bp product corresponding to T. cruzi, no amplification was observed for T. vivax or T. evansi (Figure 3).
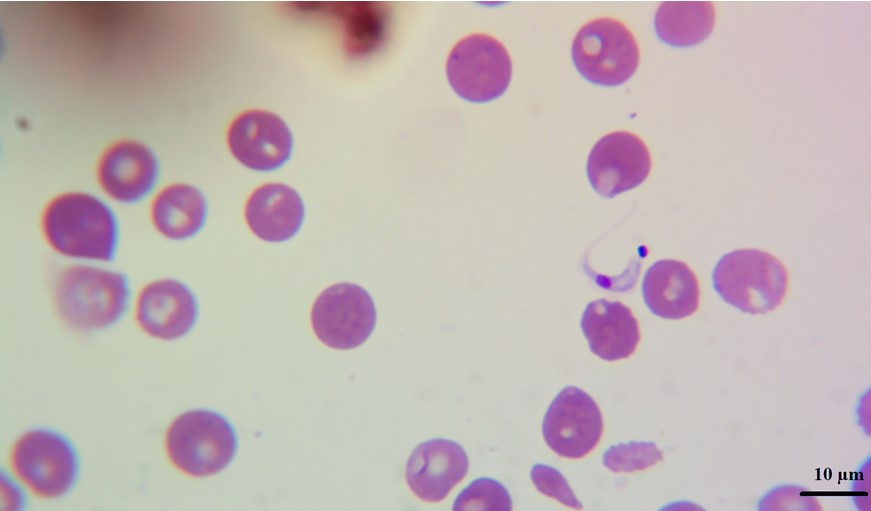
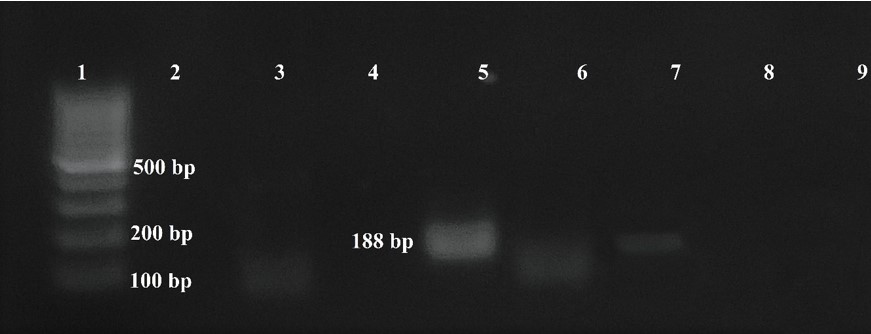
Infected dogs with T. cruzi are a potential risk factor in the transmission of human T. cruzi infections (Vasconcelos et al., 2021), since they move or stay in areas where vectors normally inhabit, in addition to the fact that dogs are infected in the same way as humans are. It has also been observed that dogs chew the insect and at least this route of transmission is experimentally highly effective for laboratory animals, domestic or wild, which indicates that some of them, under natural conditions, will be infected more easily and that this mechanism should be considered as epidemiological important (Montenegro et al., 2002).
In experimental models limited to Chagas disease in dogs, acute clinical signs are detected between 14 and 21 days after exposure and manifest with lethargy, generalized lymphadenopathy, pale mucous membranes, slow capillary filling time, ascites, weak pulse, enlarged liver, enlarged spleen and sudden death, also during this stage, arrhythmias and conduction abnormalities can be detected in infected dogs (Meyers et al., 2019); it is worth mentioning that most of these clinical signs were observed in this case, excluding sudden death.
Although there are several reports of trypanosomiasis in humans and some in dogs in Nicaragua, the importance of this case is that, for the first time in the country, T. cruzi has been identified through a direct detection assay. Other researchers suggest that PCR-positive dogs can be found although they are considered seronegative, highlighting the limitations of current serological tests infecciones (Elmayan et al., 2019) . Dogs with positive PCR and negative serology can also correspond to acute cases, although their number is higher than the estimated incidence of new infections (Elmayan et al., 2019). However, both serology and PCR should be used together since in a study carried out in the biological reserve of Bosawas in northern Nicaragua, 9% of the dogs were seropositive, but any positive result was obtained by PCR. Possible reasons could be the absence of hunting dogs, no active T. cruzi infection, or potential degradation of the sample (Roegner et al., 2019). Unlike Nicaragua, in the neighboring country of Costa Rica, trypanosomiasis in canines has been better documented with results from both serological (Bonilla et al., 2018) and molecular tests (Bonilla et al., 2019). Similar results can be expected in both countries since the environmental conditions and vector distribution are comparable (Peterson et al., 2019).
This case could raise awareness in the country about the importance of reporting canine trypanosomiasis as part of epidemiological surveillance programs to control human cases of Chagas disease (Kumar, 2017). Furthermore, it is useful to advertise veterinary professionals to apply more sensitive tests to detect possible T. cruzi infections in domestic animals.
Data availability
All data are available on request from the authors.
Conflicts of interest
The authors declare that they have no competing interests.
References
Birhanu, H., Fikru, R., Said, M., Kidane, W., Gebrehiwot, T., Hagos, A., Alemu, T., Dawit, T., Berkvens, D., Goddeeris, B. M., & Büscher, P. (2015). Epidemiology of Trypanosoma evansi and Trypanosoma vivax in domestic animals from selected districts of Tigray and Afar regions, Northern Ethiopia. Parasites & Vectors, 8(1), 212. https://doi.org/10.1186/s13071-015-0818-1
Bonilla, M. C., Castro-Vásquez, R. M., Herrero-Acosta, M. V., Urbina-Villalobos, A., & Dolz, G. (2019). Canine trypanosomiasis in an endemic Costa Rican community: Demonstration of the active infection cycle. Veterinary Parasitology: Regional Studies and Reports, 17, 100307. https://doi.org/10.1016/j.vprsr.2019.100307
Bonilla, M. C., Herrero-Acosta, M. V., Urbina-Villalobos, A., & Dolz, G. (2018). Detección de anticuerpos contra Trypanosoma cruzi en caninos de Costa Rica. Ciencias Veterinarias, 36(2), 1–14. https://doi.org/10.15359/rcv.36-2.1
Elmayan, A., Tu, W., Duhon, B., Marx, P., Wolfson, W., Balsamo, G., Herrera, C., & Dumonteil, E. (2019). High prevalence of Trypanosoma cruzi infection in shelter dogs from southern Louisiana, USA. Parasites & Vectors, 12(1), 322. https://doi.org/10.1186/s13071-019-3572-y
Fikru, R., Hagos, A., Rogé, S., Reyna-Bello, A., Gonzatti, M. I., Merga, B., Goddeeris, B. M., & Büscher, P. (2014). A Proline Racemase Based PCR for Identification of Trypanosoma vivax in Cattle Blood. PLoS ONE, 9(1). https://doi.org/10.1371/journal.pone.0084819
Kumar, S. (2017). Trypanosomosis in dog: A case report. Exploratory Animal And Medical Research, 7(2), 220–222.
Lizundia, R., Picado, A., Cordero, M., Calderón, A., Deborggraeve, S., Montenegro, V. M., & Urbina, A. (2014). Molecular and serological rapid tests as markers of Trypanosoma cruzi infection in dogs in Costa Rica. Tropical Parasitology, 4(2), 111. https://doi.org/10.4103/2229-5070.138539
Meyers, A. C., Hamer, S. A., Matthews, D., Gordon, S. G., & Saunders, A. B. (2019). Risk factors and select cardiac characteristics in dogs naturally infected with Trypanosoma cruzi presenting to a teaching hospital in Texas. Journal of Veterinary Internal Medicine, 33(4), 1695–1706. https://doi.org/10.1111/jvim.15516
Montenegro, V. M., Jiménez, M., Dias, J. P., & Zeledón, R. (2002). Chagas Disease in Dogs from Endemic Areas of Costa Rica. Memórias Do Instituto Oswaldo Cruz, 97(4), 491–494. https://doi.org/10.1590/S0074-02762002000400006
Moser, D. R., Kirchhoff, L. V., & Donelson, J. E. (1989). Detection of Trypanosoma cruzi by DNA amplification using the polymerase chain reaction. Journal of Clinical Microbiology, 27(7), 1477–1482. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC267598/
Peterson, J. K., Hashimoto, K., Yoshioka, K., Dorn, P. L., Gottdenker, N. L., Caranci, A., Stevens, L., Zuniga, C., Saldaña, A., Rodriguez, S., & Monroy, C. (2019). Chagas Disease in Central America: Recent Findings and Current Challenges in Vector Ecology and Control. Current Tropical Medicine Reports, 6(2), 76–91. https://doi.org/10.1007/s40475-019-00175-0
Roegner, A. F., Daniels, M. E., Smith, W. A., Gottdenker, N., Schwartz, L. M., Liu, J., Campbell, A., & Fiorello, C. V. (2019). Giardia Infection and Trypanosoma Cruzi Exposure in Dogs in the Bosawás Biosphere Reserve, Nicaragua. EcoHealth, 16(3), 512–522. https://doi.org/10.1007/s10393-019-01434-2
Sirois, M. (2018). Laboratory Procedures for Veterinary Technicians E-Book.
Vasconcelos, L. A. S. de, Oliveira, J. C., Silva Junior, R. C. A. da, Justiniano, S. C. B., Souza, É. dos S., Magalhães, L. K. C., Silveira, H., Silva, G. A. V. da, Guerra, J. A. de O., Guerra, M. das G. V. B., Vasconcelos, L. A. S. de, Oliveira, J. C., Silva Junior, R. C. A. da, Justiniano, S. C. B., Souza, É. dos S., Magalhães, L. K. C., Silveira, H., Silva, G. A. V. da, Guerra, J. A. de O., & Guerra, M. das G. V. B. (2021). Trypanosoma cruzi discrete typing unit TcIV implicated in a case of acute Chagas disease in a domiciliated dog in the western Amazon. Revista Da Sociedade Brasileira de Medicina Tropical, 54. https://doi.org/10.1590/0037-8682-0873-2020
WHO, W. H. (2015). Chagas disease in Latin America: An epidemiological update based on 2010 estimates = Maladie de Chagas en Amérique latine : le point épidémiologique basé sur les estimations de 2010. Weekly Epidemiological Record = Relevé Épidémiologique Hebdomadaire, 90(06), 33–44. https://apps.who.int/iris/handle/10665/242316
Author notes
byronfloressomarriba@gmail.com

