Articulos
Effect of pH and Ttemperature on photocatalytic oxidation of methyl orange using black sand as photocatalyst
Efecto del pH y la temperatura sobre la oxidación fotocatalítica de naranja de metilousando arena negra como fotocatalizador
Effect of pH and Ttemperature on photocatalytic oxidation of methyl orange using black sand as photocatalyst
Revista tiempo&economia, vol. 1, no. 1, 2017
Universidad de Bogotá Jorge Tadeo Lozano
Received: 02 August 2017
Accepted: 07 December 2017
Abstract: Azo dyes are considered hazardous compounds for the environment and human health. Methyl orange is one type of azo dyes and it is widely used in textile, leather, and other chemical industries. The degradation of this compound is a challenge for traditional treatments. Advanced oxidation processes such as heterogeneous photocatalysis, sonolysis, radiolysis, etc., become an alternative for mineralizing organic compounds by producing a highly oxidant agent (OH•). Nowadays, a great number of research studies have tried to modify TiO2 with metals in order to improve the degradation of hazardous pollutants such as azo dyes. This study used black sand as an alternative photocatalyst, evaluating the influence of pH (2, 5, 3 and 8) and temperature (20, 25, 30 and 35°C) on the photocatalytic oxidation of methyl orange. Black sand was magnetically separated. The fraction that showed the best characteristics for dye degradation was used. Experimental results allowed establishing that methyl orange photocatalytic oxidation is best performed at pH 2 and 30°C, with a degradation percentage of 96.93%. The reaction follows a pseudo-first order kinetic. In addition, the kinetic coefficient found at different temperatures was correlated using Arrhenius equation in order to determinate changes in the kinetic coefficient depending on the temperature. The equation pre-exponential coefficient was 374782115.1 and energy activation was -58,104.4 J mol-1 K-1.
Keywords: Azo dyes, kinetic evaluation, heterogeneous photocatalysis, pH and temperature influence.
Resumen: Los colorantes azo son considerados compuestos peligrosos para el medio ambiente y la salud humana. Uno de estos compuestos es el naranja de metilo el cual ha sido ampliamente utilizado en industrias textiles, de cuero y otras industrias químicas. La degradación de este compuesto se ha convertido en un reto mediante tratamientos convencionales. Algunos procesos de oxidación avanzada como la fotocatálisis heterogénea, la sonólisis, la radiólisis, etc., constituyen una alternativa para la mineralización de compuestos orgánicos gracias a la generación in-situ de un radical altamente oxidante (OH•). En la actualidad, un gran número de investigaciones han tratado de modificar el TiO2 con metales para mejorar la degradación de contaminantes peligrosos como los colorantes azo. En este estudio se utilizó arena negra como una alternativa de fotocatalizador para evaluar el efecto del pH (2,0, 5,8 y 8,0) y la temperatura (20, 25, 30 y 35 °C) en el proceso de oxidación fotocatalítica del naranja de metilo. Debido a las características del mineral, se aplicaron diferentes campos magnéticos para obtener fracciones que fueron utilizadas como semiconductor, de las cuales se utilizó la que exhibió mejores condiciones para la degradación del colorante. Los resultados experimentales permitieron determinar que la oxidación fotocatalítica de naranja de metilo presentó una mayor eficiencia con un pH 2.0 y a 30° C, con un porcentaje de degradación de 96.93 %. La reacción observada describió una cinética de pseudo primer orden y mediante la ecuación de Arrhenius se determinó el coeficiente cinético a diferentes temperaturas. El coeficiente pre exponencial de la ecuación fue de 374782115,1 y la energía de activación fue de -58104,4 J mol-1 K-1.
Palabras clave: colorantes azo, evaluación cinética, fotocatálisis heterogénea, influencia del pH y la temperatura.
INTRODUCTION
Hazardous compounds such as azo dyes are knownto be a major problem for the environment sincetheir degradation process is not easily carried out bymeans of traditional treatments. Methyl orange (mo) is an azo dye considered as one of the main waterpollutants (Figueroa, Vázquez & Alvarez-Gallegos,2009; Hai, Yamamoto, Nakajima, & Fukushi, 2011;Martínez-Huitle & Brillas, 2009; Muda et al., 2010).Produced for textile, leather and pharmaceuticalindustries, the degradation of this compound cab beeasily performed by reduction at cathode because ofits positive high redox potential (Feng et al., 2009).
Photocatalytic processes have emerged with severaladvantages for organic wastewater treatment dueto the complete destruction or mineralization of pollutants to carbon dioxide and inorganic constitutesin water and gas phases (Chen, Tsai & Huang, 2005;Wang, Zheng, Xu & Li, 2011). The main photocatalystused in these processes is TiO2 due to its low price,availability, non-photo-corrosion, suitable band gapenergy and chemical stability (Haarstrick, Kut & Elmar,1996; Matos, Laine & Herrmann, 2001). Nevertheless, the use of this semiconductor has presented somelimitations for these treatments, considering theamount of energy required when using uv lamps orhaving a doped semiconductor with metals, such asgold (Cheng et al., 2016; Oros-Ruiz et al., 2012).
Heterogeneous catalysis on mineral surfaces is partof advanced oxidation processes and constitutes analternative to degrade polluting compounds of watersources (Schoonen, Xu & Strongin, 1998), consideringthat this process stimulates the mineral activation inside the visible spectrum of electromagnetic radiationand improves stability against photo-corrosion. Theuse of minerals as photocatalyst materials has beenapplied to the degradation of aromatic and phenoliccompounds (Sonawane & Dongare, 2006) and oxalicacid (Iliev et al., 2006), among others, with relativelysatisfactory results (Arshadi et al., 2016).
The use of the mineral conglomerate known in thisstudy as black sand (bs) has not been a commonpractice in this type of studies. In contrast, this materialhas been widely used in applications in the field ofmetallurgy. bs is composed by iron oxides, calcium,magnesium and silicon (Vargas & Forero, 2011),whose properties foster its use as a photocatalyst. Aprevious study showed that the magnetic separationof bs recovered from Colombian beaches (SantaMarta), named M1, M2, nm (Non-magnetic) and rm(raw material), was highly effective, showing higherdiscoloration percentages in mo compared to rawmaterials (Acosta, Ibatá & López, 2016). The mosteffective fraction was M2, which was exposed to amagnetic field of 0.1645 T. Therefore, pH influencein the solution is a variable to consider, since itaffects dyes’ absorption capacity, absorbent surfaceand solubility. It is important to emphasize that thediscoloration process of mo works better at acid pH,and decreases its efficacy at alkaline pH. This becausethe absorbent surface increases its positive chargewith acid pH and mo molecules reach its maximumpositive charge, favoring electrostatic absorption (Subbaiah & Kim, 2016).
The effectiveness and speed of every chemicalreaction is directly influenced by temperature (Lien& Zhang, 2007). As an example, high temperaturescan accelerate the speed of mo molecules, possiblyleading to an increase in absorption efficiency. However, when temperature exceeds 65°C efficiencydecreases as a result of a week intermolecularattraction of absorbent materials (Arshadi et al.,2016). Previous studies about mo degradation haveshown that kinetic degradation of mo is describedby a pseudo-first model (Lee et al., 2015; Li, Li, Li &Yin, 2006; Yan et al., 2016). In that sense, this studyis carried out in order to find optimum conditions forthe photocatalytic oxidation process of mo using bsas semiconductor and pH and temperature as processvariables. In addition, we study the kinetic tendencyof mo degradation at different temperatures.
MATERIALS AND METHODS
Material Black Sand
The material was separated in four fractions bymagnetic fields in order to make the best use ofits high iron content, as suggested in a previousstudy (Acosta, Ibatá & López, 2016). The fractionthat was used (M2) in the experimental phase was magnetically separated by a magnetic field of 0.164T;this fraction was used because of its composition. M2 fraction was evaluated to identify its chemical (xrf),morphological (sem micrographs) and structural (xrd) characterization. Additionally, an edx analysiswas carried out with the purpose of determining itselemental composition.
Photocatalytic oxidation of methylorange
M2 fraction was evaluated through the photocatalytic oxidation of 150 mL of mo to 20 mg L-1 in aqueous suspension with a bs dosage of 1.0 g L-1. This reaction was carried out in an immersed well quartzphotoreactor (Ace Glass Inc.) equipped with a cooling tube. Besides, 10 mmol of H2O2 were added to improvethe photocatalytic activity of the mineral (Gao, Yang,Hu & Zhang, 2004; He et al., 2015; Sahel et al., 2016). In order to study the effect of the initial pH solution,the value was adjusted by adding of HCl and/or NaOH solutions. For these case, were tested at pH 2.0, monatural pH (5.8) and 8.0. The experiments were carriedout at 20, 25, 30 and 35°C. Reactions were carried outduring three hours under uv-Vis irradiation (λ=310nm) in magnetic stirring with 30 minutes of nonillumination,in order to reach adsorption-desorption equilibrium. mo samples were withdrawn from thereactor with a semiconductor, which was in turn removed from the liquid phase by thermo scientific centrifuge. Kinetic degradation of mo samples wasevaluated by measuring mo signal disappearance every 30 minutes with a UV-Vis Shimadzu 2600 spectrophotometer. An evaluation of kinetic degradation for each reaction was performed using the coefficient of degradation as a way to determinethe treatment with the highest speed reaction. The concentration of the substrate was measured at the beginning (Co) and end of the treatment (Cf).
RESULTS AND DISCUSSION
Chemical characterization of M2 fraction
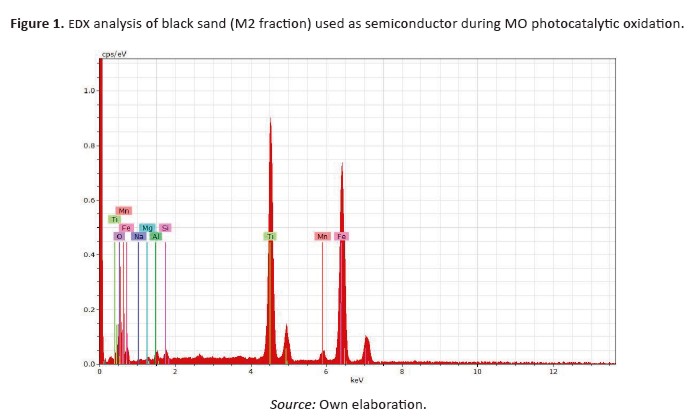
The results obtained through X-ray fluorescence analysis showed that the mineral presents are presentative percentage of iron oxides such as Fe2O3 (50.313 %w/w), with a slight difference with the percentage of TiO2 (37.027 %w/w) (G.A. ReyesGomez, 2015). Figure 1 shows the results of X-ray energy dispersive analysis (VEGA3 TESCAN) for the elemental composition by weight percent of M2 fraction (used as semiconductor for photocatalytic experiments), which it is mainly composed by iron(32.89%), titanium (26.74%), aluminium (1.18%) andoxygen (39.67%). The high amount of iron in thesample could be the main reason of the discolorationof mo, since iron could help doping TiO2 naturally,improving its photocatalytic activity.
Morphological and structural characterization of M2 fraction
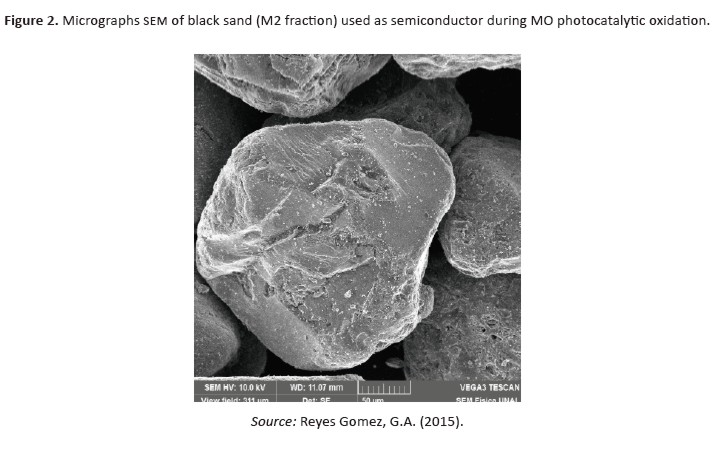
In order to determinate the morphological structure of M2 fraction, we carried out an analysis with scanning electron microscopy (VEGA3 TESCAN), which showedthat the mineral fraction is composed by irregular, rounded and semi-rounded grains (Figure 2).
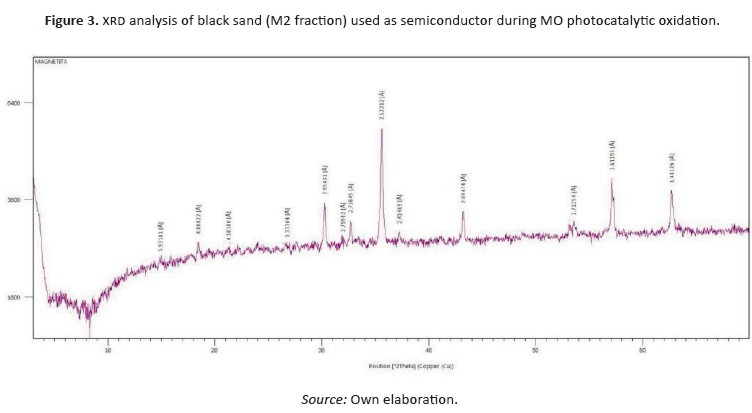
X-ray diffraction analysis (X-ray powder diffractometer PANalytical X’Pert Pro PW 3064/60) with Cu-Kαradiation, was carried out to evaluate the crystalline phases of M2 fraction (Figure 3). This fraction was characterised for its content of iron oxides in the form of magnetite Fe3O4 (~ 99.0 %), showing a few amount of zircon, pyroxenes, altered grains and silicates (suchas quartz), that might help improve the photocatalyticmechanism for mo photocatalytic oxidation.
MO photocatalytic oxidation
MO photocatalytic oxidation experiments were carried out with two process variables (pH and temperature), while the response variable was theremoval percentage of MO.
Influence of pH
Table 2 shows the results of initial and final conditions of each experiment.
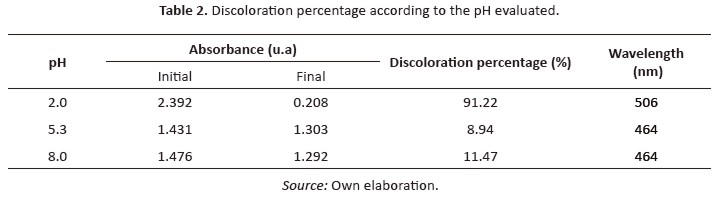
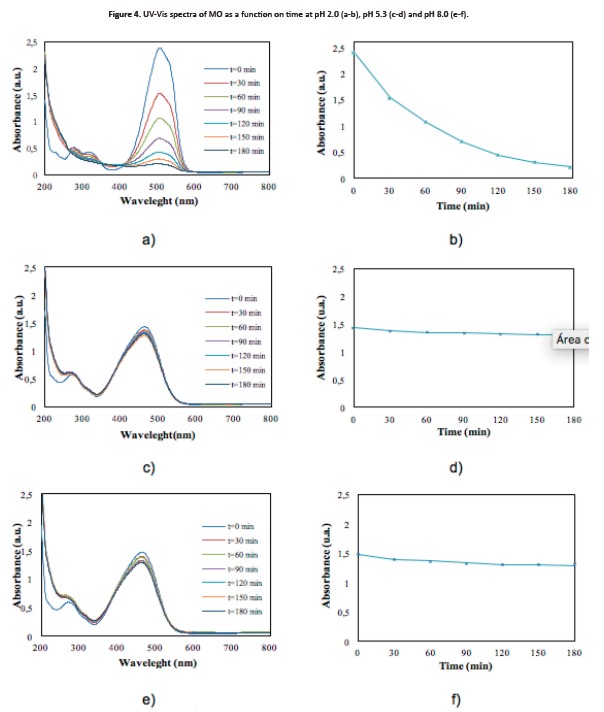
The absorption capacity of materials decreases whenthe pH of the solution increases, which means that the absorption process is possibly carried out with high effectiveness to acid pH, thus increasing the efficiency of the material (Subbaiah & Kim, 2016). Figure 4 (a-b) shown the uv-Vis spectra for the photocatalytic oxidation of mo as a time function. At the beginning of the reaction, the degradation showed a higher speed than at the end. Nonetheless, it is possibleto state it is necessary to allow the reaction timeup to 180 min (or even more) in order to obtain the highest possible discoloration of mo. On the contrary, when pH increases, the photocatalytic oxidation of mo is less effective (Figure 4c-4f). This means that the mo photocatalytic oxidation was favored at acid conditions, while at alkaline conditions, the process is governed by hydrolysis (Arshadi et al., 2016; Dagar &Narula, 2016).
Influence of temperature
Once the optimal pH condition for mo photocatalytic oxidation was determined, the effect of temperatureof solution was tested. According to literature, thisprocess is more efficient when temperature does notexceed 65°C. On the other hand, H2O2 is thermallydecomposed into oxygen and water at higher temperature, eventually scavenging the production of reactive radicals (Herney-Ramirez, Vicente & Madeira,2010) however, the reaction is less efficient whentemperature is below 25°C (Arshadi et al., 2016).
Figure 5 shows the mo photocatalytic degradation as function of solution temperature. A slight discoloration percentage can be appreciate with the temperature increase however, this maximum can be obtained at less time reaction (Figure 5e-f)
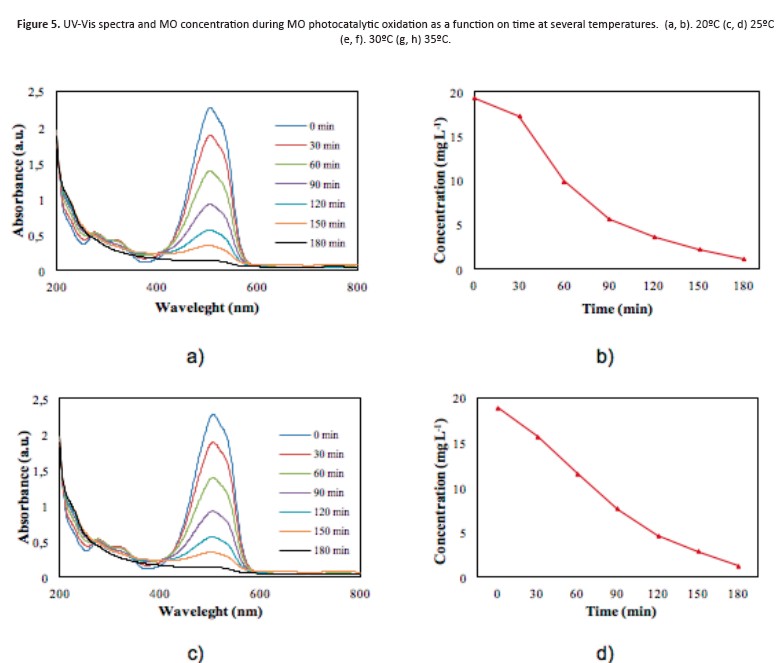
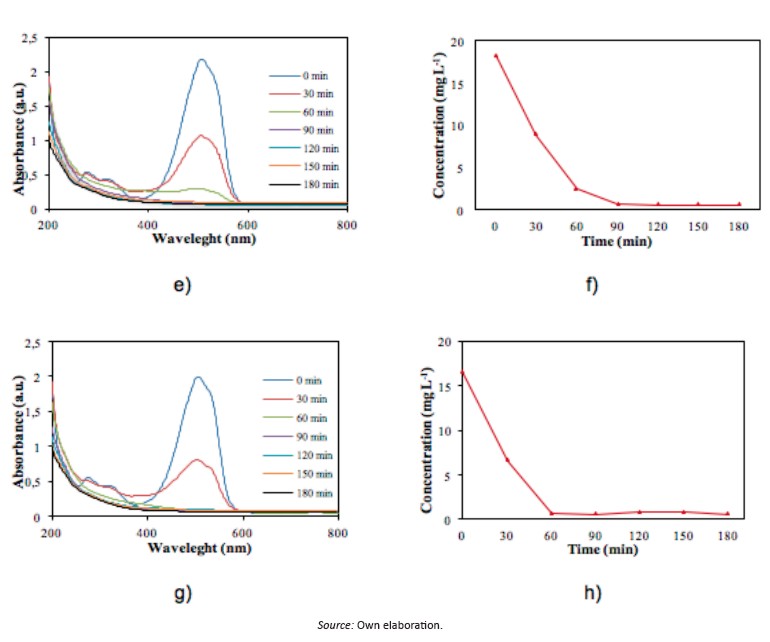
About concentration, mo photocatalytic oxidation exhibits several profiles of discoloration, reaching maximum values at different temperatures promoting a reduction in the reaction time, decreasing the operational costs in the process, especially in energy consumption for irradiation.
Kinetic studies for MO photocatalyticoxidation at different temperatures
MO photocatalytic oxidation data were adjusted atpseudo-first order kinetic model. This goes in line with results of previous research studies on the subject (Lee et al., 2015; Matouq et al., 2014). The lineal correlation of the results was performed in order to calculate the kinetic coefficients Kapp of the reactionsat different temperatures, according to Eq. 1 (Dagar &Narula, 2016).
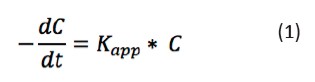 [Eq 1]
[Eq 1]The linearized form (Eq. 2) produces where Kapp is the slope of the graph, y axis is the natural logarithm of the quotient between the final and initialconcentration, and x axis is the reaction time.
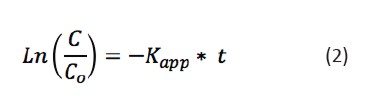 [Eq 2]
[Eq 2]Figure 6 shows the linearized form of the kinetic data analysed. When temperature increased, the stable condition was reached in less time. The highest removal percentage was reached at 30°C. A slight decrease in the effectiveness of the reaction for mode gradation was observed at 35°C. As mentioned, the values used to construct the graphs were correlated to establish the value of the kinetic coefficient.
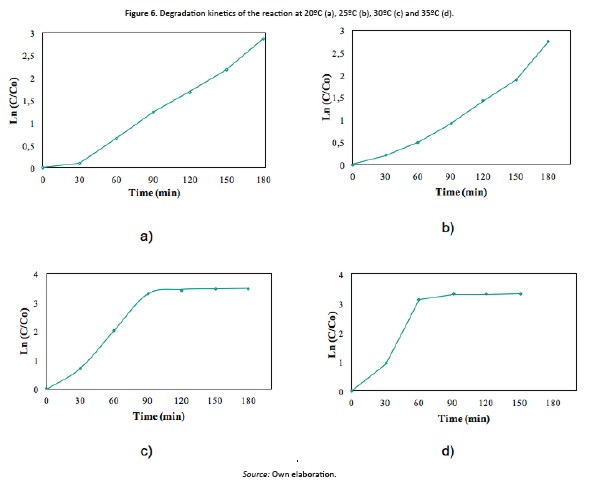
Table 3 presents the values of the coefficients calculated at different temperatures, which increases with temperature solution. Once kinetics coefficients and temperature were identified, we calculated the variation of the kinetic coefficient with the temperature using Svante Arrhenius equation (Dahm & L. Brezonik, 1995), whose linearized form is represented by Eq. 3.
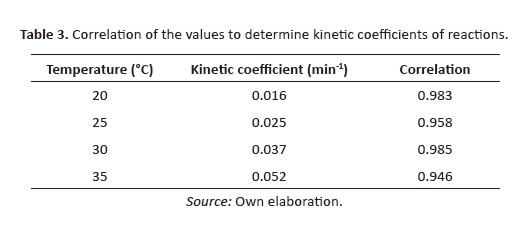
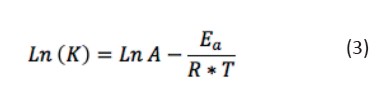 [Eq 3]
[Eq 3]Where Ea is the activation energy (J mol-1), A the pre exponential factor of Arrhenius, R the gas constant (8,314472 J mol-1 K-1), and T is temperature (K). Figure 7 shown the linearization of (3) for the determinationof kinetic constants.
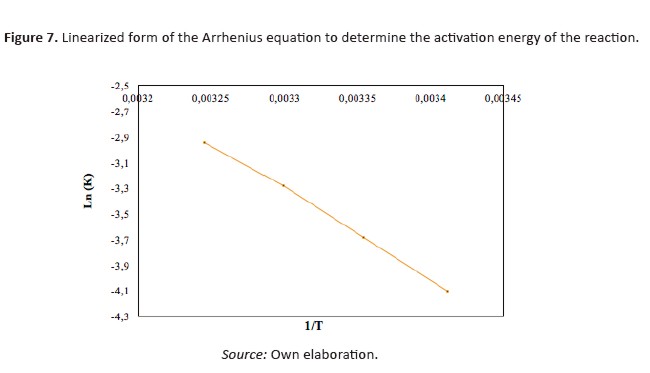
The value obtained for the activation energy of mo degradation was -58104.41 J mol-1. The pre exponential coefficient was 374782115.1 min-1, with a statistical correlation coefficient of R2 0.998. Finally, Eq. 4 allows calculating the kinetic coefficient as a function ontemperature for MO photocatalytic oxidation
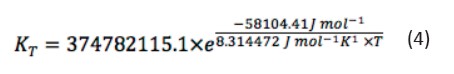 [Eq 4]
[Eq 4]CONCLUSIONS
Results of the activation energy determined mo photocatalytic oxidation. It is concluded that bs couldbe used as an alternative semiconductor with better economic benefits for the degradation process oforganic compounds, compared with the activation energy using TiO2 as semiconductor (44.1 kJ mol-1) (Arshadi et al., 2016). These results allow concluding that optimal conditions for higher removal efficiency of mo (96.93%) under uv irradiation are: 1.0 g L-1dosage of bs, pH 2 and 30°C, with a reaction time of 90 min. It is important to mention that at 35°C the reaction time was 60 min, with a slightly higher removal percentage (96.339%).
References
Acosta, P., Ibatá, A., & López, A. (2016). Evaluation of the Discoloration of Methyl Orange Using Black Sand as Semiconductor through Photocatalytic Oxidation and Reduction. International Scholarly and Scientific Research & Innovation, 10(10),
Arshadi, M., Abdolmaleki, M. K., Mousavinia, F.,Khalafi-Nezhad, A., Firouzabadi, H., & Gil, A. (2016).Degradation of methyl orange by heterogeneousFenton-like oxidation on a nano-organometalliccompound in the presence of multi-walled carbonnanotubes. Chemical Engineering Researchand Design, 112(Supplement C), 113-121.doi:10.1016/j.cherd.2016.05.028.
Chen, L. C., Tsai, F. R., & Huang, C. M. (2005).Photocatalytic decolorization of methyl orangein aqueous medium of TiO2 and Ag–TiO2immobilized on γ-Al2O3. Journal of Photochemistryand Photobiology A: Chemistry, 170(1), 7-14.doi:10.1016/j.jphotochem.2004.07.012.
Cheng, X. Q., Ma, C. Y., Yi, X. Y., Yuan, F., Xie, Y., Hu, J. M.,… & Zhang, Q. Y. (2016). Structural, morphological,optical and photocatalytic properties of Gd-dopedTiO2 films. Thin Solid Films, 615(Supplement C),13-18. doi:10.1016/j.tsf.2016.06.049.
Dagar, A., & Narula, A. K. (2016). Photo-degradationof methyl orange under visible light by PEDOT/NiO/Fly ash cenosphere. Materials Chemistryand Physics, 183(Supplement C), 561-570.doi:10.1016/j.matchemphys.2016.09.015.
Dahm, C., & L. Brezonik, P. (1995). Chemical Kineticsand Process Dynamics in Aquatic Systems. Journalof the North American Benthological Society, 14,354.
Feng, C., Liu, L., Li, F., & Li, X. (2009). Microbial fuelcell with an azo-dye-feeding cathode. AppliedMicrobiology and Biotechnology, 85, 175-183.
Figueroa, S., Vázquez, L., & Alvarez-Gallegos, A. (2009). Decolorizing textile wastewater with Fenton’s reagent electrogenerated with a solar photovoltaic cell. Water Research, 43(2), 283-294.doi:10.1016/j.watres.2008.10.014.
Gao, Y., Yang, M., Hu, J., & Zhang, Y. (2004). Fenton’s process for simultaneous removal of TOC and Fe2+ from acidic waste liquor. Desalination, 160,123-130.
Hai, F. I., Yamamoto, K., Nakajima, F., & Fukushi, K.(2011). Bioaugmented membrane bioreactor (MBR) with a GAC-packed zone for high rate textile waste water treatment. Water Research, 45(6),2199-2206. doi:10.1016/j.watres.2011.01.013.
He, H., Zhong, Y., Liang, X., Tan, W., Zhu, J., & Wang,C. Y. (n.d.). Natural Magnetite : an efficient catalystfor the degradation of organic contaminant. Nature Publishing Group, 1-10.
Herney-Ramirez, J., Vicente, M. A., & Madeira,L. M. (2010). Heterogeneous photo-Fentonoxidation with pillared clay-based catalysts forwastewater treatment: A review. Applied CatalysisB: Environmental, 98(1), 10-26. doi:10.1016/j.apcatb.2010.05.004.
Iliev, V., Tomova, D., Todorovska, R., Oliver, D., Petrov,L., Todorovsky, D., & Uzunova-Bujnova, M. (2006). Photocatalytic properties of TiO2 modified withgold nanoparticles in the degradation of oxalicacid in aqueous solution. Applied CatalysisA: General, 313(2), 115-121. doi:10.1016/j.apcata.2006.06.039.
Lee, H. J., Kim, J. H., Park, S. S., Hong, S. S., & Lee, G.D. (2015). Degradation kinetics for photocatalyti creaction of methyl orange over Al-doped ZnO nanoparticles. Journal of Industrial and Engineering Chemistry, 25 (Supplement C), 199-206. doi:10.1016/j.jiec.2014.10.035.
Li, Y., Li, X., Li, J., & Yin, J. (2006). Photocatalyticdegradation of methyl orange by TiO2-coatedactivated carbon and kinetic study. WaterResearch, 40(6), 1119-1126. doi:10.1016/j.watres.2005.12.042.
Lien, H. L., & Zhang, W. X. (2007). Nanoscale Pd/Febimetallic particles: Catalytic effects of palladium on hydrodechlorination. Applied Catalysis B:Environmental, 77(1), 110-116. doi:10.1016/j.apcatb.2007.07.014.
Martínez-Huitle, C. A., & Brillas, E. (2009).Decontamination of waste waters containing synthetic organic dyes by electrochemicalmethods: A general review. Applied Catalysis B: Environmental, 87(3), 105-145. doi:10.1016/j.apcatb.2008.09.017.
Matos, J., Laine, J., & Herrmann, J. M. (2001). Effect of the Type of Activated Carbons on the Photocatalytic Degradation of Aqueous Organic Pollutants by UV Irradiated Titania. Journal of Catalysis, 200(1), 10-20. doi:10.1006/jcat.2001.3191.
References
Matouq, M., Al-Anber, Z., Susumu, N., Tagawa, T., & Karapanagioti, H. (2014). The kinetic of dyes degradation resulted from food industry in waste water using high frequency of ultrasound. Separation and Purification Technology, 135(Supplement C), 42-47. doi: 10.1016/j.seppur.2014.08.002
Muda, K., Aris, A., Salim, M. R., Ibrahim, Z., Yahya, A.,van-Loosdrecht, M. C. M., … & Nawahwi, M. Z.(2010). Development of granular sludge for textile waste water treatment. Water Research, 44(15),4341-4350. doi:10.1016/j.watres.2010.05.023.
Oros-Ruiz, S., Gómez, R., López, R., Hernández-Gordillo, A., Pedraza-Avella, J. A., Moctezuma,E., & Pérez, E. (2012). Photocatalytic reduction of methyl orange on Au/TiO2 semiconductors. Catalysis Communications, 21 (Supplement C), 72-76. doi:10.1016/j.catcom.2012.01.028.
Reyes Gomez, G.A. (2015). Producción fotocatalítica de hidrógeno basada en el mineral arena negra, Universidad Libre sede Principal, https://repository.unilibre.edu.co/handle/10901/9974?show=full.
Sahel, K., Elsellami, L., Mirali, I., Dappozze, F., Bouhent,M., & Guillard, C. (2016). Hydrogen peroxide and photocatalysis. Applied Catalysis B: Environmental, 188(Supplement C), 106-112. doi:10.1016/j.apcatb.2015.12.044.
Schoonen, M. A. A., Xu, Y., & Strongin, D. R. (1998).An introduction to geocatalysis. Journal of Geochemical Exploration, 62(1), 201-215.doi:10.1016/S0375-6742(97)00069-1.
Sonawane, R. S., & Dongare, M. K. (2006). Sol-gelsynthesis of Au/TiO2 thin films for photocatalytic degradation of phenol in sunlight. Journal of Molecular Catalysis A: Chemical, 243(1), 68-76. doi:10.1016/j.molcata.2005.07.043.
Subbaiah, M. V., & Kim, D. S. (2016). Adsorption of methyl orange from aqueous solution by aminated pumpkin seed powder: Kinetics, isotherms, and thermodynamic studies. Ecotoxicology and Environmental Safety, 128 (Supplement C), 109-117. doi:10.1016/j.ecoenv.2016.02.016.
Vargas, J. A., & Forero, A. H. (2011). Obtención de hierro a partir de arenas negras del Atlántico colombiano. Desembocadura río Magdalena. Revista de la Facultad de Ingeniería, 26, 19-26.
Wang, E., Zheng, Q., Xu, S., & Li, D. (2011). Treatmentof Methyl Orange by Photocatalysis Floating Bed. Procedia Environmental Sciences, 10(Part B), 1136-1140. doi:10.1016/j.proenv.2011.09.181.
Yan, J., Zhu, Y., Qiu, F., Zhao, H., Yang, D., Wang, J., & Wen,W. (2016). Kinetic, isotherm and thermodynamic studies for removal of methyl orange using a novelβ-cyclodextrin functionalized graphene oxideisophoronediisocyanate composites. Chemical Engineering Research and Design, 106 (SupplementC), 168-177. doi:10.1016/j.cherd.2015.12.023