



Cartas al editor
Defining a good research question using the PICOT and FINGER format
Definiendo una buena pregunta de investigacion usando el formato PICOT y FINGER
Gaceta Médica Boliviana
Universidad Mayor de San Simón, Bolivia
ISSN: 1012-2966
ISSN-e: 2227-3662
Periodicity: Semestral
vol. 43, no. 2, 2020
Received: 05 October 2020
Accepted: 02 December 2020

In Bolivia, research is poorly standardised1-3, starting with the research question that is reflected in what is described in the objectives of the study4, in original articles or review articles, which are the two types of papers that must report their general objective in the last paragraph of the introduction section, which is why we want to introduce the PICOT5,6;and FINGER format6, both internationally standardised, in books and scientific articles, focused on teaching research in health sciences, to generate good questions for research, and thus good scientific articles.
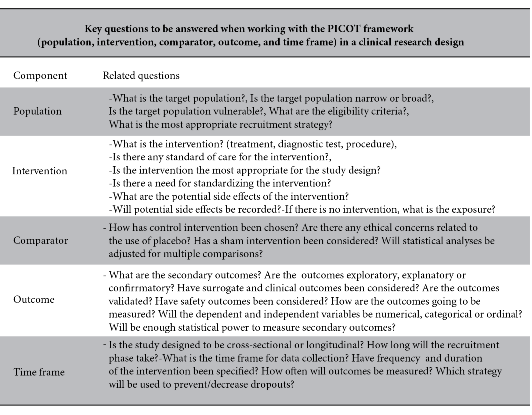
To highlight the following: if research questions are formulated using the PICOT and FINGER7, format; the type of study, universe, sampling calculation, type of sampling, inclusion and exclusion criteria, study variables, statistical analysis and ethical considerations can be correctly identified, and similar articles can be searched to support the study that is to be carried out5.
For all that mentioned, the following should be emphasised, the PICOT format 5,7, is an acronym indicating necessary elements that the research question should contain: P (Population: Helps to identify Universe and Sample) , I(Intervention and/or Exposure: Describes whether the research will be with intervention or observation of the study event.), C (Comparison: Identifies whether there will be two or more groups to be evaluated in relation to the type of study, not always mandatory to describe, especially in cross-sectional studies.), O (Outcome: Describes how the results will be measured.), T(Time: States the time spent on the research.).
Table 1 summarises the most frequently asked questions that the researcher should answer if using the PICOR format to generate research questions.
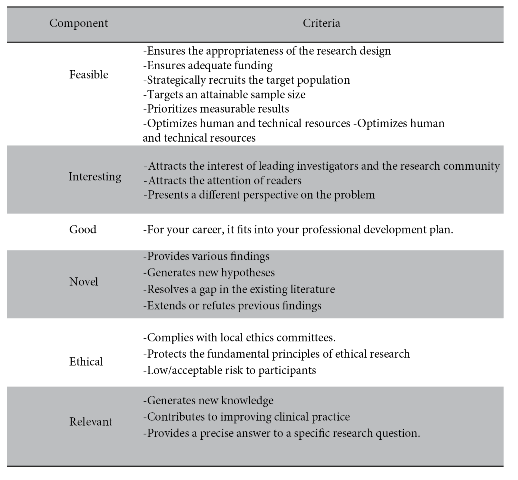
The FINGER format6,7is an acronym that helps to elucidate whether the research question is: F (Feasible: indicating whether it will have the participants, human and financial resources and reasonable time to be completed), I (Interesting: the interest that the scientific community could have in the proposed study), N (Novel: new scientific findings), G (Good: enhances career/line of research), E (Ethical: ensuring that the risk to participants is low/acceptable, this always reviewed and endorsed by a team of ethics experts), E (Ethical: ensuring that the risk to participants is low/acceptable, this always reviewed and approved by a team of ethics experts) and R (relevant: for the improvement of clinical practice and the future of research). Table 2 provides a summary and brief recommendations of the FINGER format.
Hopefully, this will open a debate about the importance of using international standards from undergraduate research to medical residencies and postgraduate health sciences 8,9 in Bolivia.
References
1. Carvajal Tapia A. E. Una visión panorámica de la productividad científica en salud de Bolivia. Rev Méd La Paz. 2017; 23(2): 88-90.[citado 29 nov 2020] Disponible en: http://www.scielo.org.bo/scielo.php?pid=S1726-89582017000200021&script=sci_arttext [ Links ]
2. Larrazabal Córdova C. La investigación y el pregrado. Gac Méd Bol. 2014; 37(1):5-5.Disponible en: http://www.scielo.org.bo/scielo.php?pid=S1012-29662014000100001&script=sci_arttext [ Links ]
3. Erostegui Revilla C, De Pardo Ghetti E, Baumann Pinto GA., Suárez Barrientos EL. Evaluación de la difusión de la producción científica en Bolivia. Gac Med Bol . 2011 ; 34( 1 ): 5-5. [citado 2020 Nov 29] Disponible en: http://www.scielo.org.bo/scielo.php?script=sci_arttext&pid=S1012-29662011000100001&lng=es. [ Links ]
4. Fandino W. Formulating a good research question: Pearls and pitfalls. Indian J Anaesth 2019 [citado 2020 nov 29];63: 611-6. Disponible en: https://www.ijaweb.org/text.asp?2019/63/8/611/264195
5. Aslam S, Emmanuel P. Formulating a researchable question: A critical step for facilitating good clinical research. Indian J Sex Transm Dis. 2010 ; 31:47-50.[citado 2020 Nov 29] Disponible en: https://www.ijstd.org/text.asp?2010/31/1/47/69003 [ Links ]
6. Patino CM, Ferreira JC. Developing research questions that make a difference. J. bras. pneumol. . 2016 [citado 2020 Nov 29]; 42 (6): 403-403. Disponible en: https://doi.org/10.1590/s1806-37562016000000354.
7. Cummings SR, Warren S. Browner. Hulley SB. Conceiving the Research Question and Developing the Study Plan. En: Hulley S.B, et al, editores. Designing Clinical Research. 4th Ed. Philadelphia. Wolters Kluwer; 2013. p. 35-47.
8. Carvajal Tapia AE, Carvajal Rodríguez E. Análisis bibliométrico de la participación estudiantil en publicaciones de artículos científicos en revistas de ciencias de la salud indizadas en SciELO Bolivia, periodo 2010-2016. Gac Med Bol . 2018; 41(1): 31-35. [citado 2020 Nov 29] Disponible en: http://www.scielo.org.bo/scielo.php?script=sci_arttext&pid=S1012-29662018000100007&lng=es. [ Links ]
9. Claros Coca Z, Claros Coca E. Producción del área de Ciencias de la Salud - SciELO Bolivia, gestión 2009-2017. Gac Med Bol . 2018; 41(1): 14-19. [citado 2020 Nov 29] Disponible en: http://www.scielo.org.bo/scielo.php?script=sci_arttext&pid=S1012-29662018000100004&lng=es. [ Links ]

