Abstract:
Dexmedetomidine is an α-agonist, widely used and popular worldwide, but little is known about its intrathecal use in Bolivian population, especially in obstetric patients.
Objectives: to evaluate the effect of intrathecal dexmedetomidine in obstetric anesthesia and observe side effects of the drug.
Methods: randomized, single-blinded clinical trial. The sample was 123 patients, each group of 41 patients fulfilling both criteria. Group B (bupivacaine + fentanyl); group D2 (bupivacaine + fentanyl+ dexmedetomidine 2 µg) and group B3 (bupivacaine + fentanyl+ dexmedetomidine 3 µg). Statistical analysis: SPSS® 25 and Excel® 2016 software were used. With a confidence level of 95% and a sampling error of 13%.
Results: motor block duration was 106.5 ± 16 minutes in group B; 142.3 ± 28.2 minutes in group D2 and 145.6 ± 16.7 minutes in group D3. Being significant between the study groups (p-value < 0.000). PAM with statistical significance before and after birth. Heart rate significant at 15, 30 and 45 minutes (p-value < 0.000, 0.001, 0.004, 0.000 and 0.002). Etilefrin in group B was 3.44 ± 1.8 ml; for group D3 it was 1.8 ± 2.7 ml and group D2 it was 0.85 ± 1.6 ml.
Conclusions: better hemodynamic stability with 2 µg dexmedetomidine, lower incidence of hypotension, use of vasopressors, few complications and an excellent state of maternal sedation.
Keywords: dexmedetomidine, caesarean section, spinal anesthesia.
Resumen:
Dexmedetomidina un α-agonista, de amplio uso y popular nivel mundial, que poco conocimiento se tiene sobre su uso de forma intratecal en la población boliviana, de manera especial en pacientes obstétricas.
Objetivos: evaluar el efecto de dexmedetomidina intratecal en anestesia obstétrica y observar los efectos secundarios del medicamento.
Métodos: ensayo clínico aleatorizado y simple ciego. La muestra fue de 123 pacientes, cada grupo de 41 pacientes cumpliendo ambos criterios. Grupo B (bupivacaina + fentanil); grupo D2 (bupivacaina + fentanil+ dexmedetomidina 2 µg) y grupo B3 (bupivacaina + fentanil+ dexmedetomidina 3 µg). Análisis estadístico: se utilizó el software SPSS® 25 y Excel® 2016. Con un nivel de confianza del 95 % y error muestral del 13 %.
Resultados: duración del bloqueo motor fue de 106,5 ± 16 minutos en el grupo B; 142,3 ± 28,2 minutos en el grupo D2 y 145,6 ± 16,7 minutos en el grupo D3. Siendo significativo entre los grupos de estudio (valor p < 0,000). PAM con significancia estadística antes y después del nacimiento. Frecuencia cardiaca significativo a los 15, 30 y 45 minutos (valor p < 0,000; 0,001; 0,004; 0,000 y 0,002). Etilefrina en el grupo B fue de 3,44 ± 1,8 ml; para el grupo D3 fue de 1,8 ± 2,7 ml y grupo D2 fue de 0,85 ± 1,6 ml.
Conclusiones: mejor estabilidad hemodinámica con 2 µg dexmedetomidina, menor incidencia de hipotensión, uso de vasopresores, las complicaciones escasas y un excelente estado de sedación materna.
Palabras clave: dexmedetomidina, cesárea, anestesia espinal.
Articulos Originales
Dexmedetomidine in spinal anesthesia for cesarean section
Dexmedetomidina en anestesia espinal para cesárea
Received: 30 October 2020
Accepted: 03 December 2020

Spinal anaesthesia is the gold standard for the management of caesarean section and with the very common complication of hypotension, caused by sympathetic vasomotor blockade accompanied by maternal symptoms such as nausea, vomiting and adverse effects on the fetus include low APGAR scores and umbilical acidosis. This has been correlated with the duration and severity of hypotension1.
The quality of spinal anaesthesia is improved by the addition of opioids and other drugs such as dexmedetomidine, clonidine, magnesium sulphate (Mg), neostigmine, ketamine and midazolam2.
Dexmedetomidine is used for sedation, analgesia, antisympathetic pharmacological effect, inhibition of tremor during the extubation phase of general anaesthesia and the only one that gives conscious sedation without respiratory depression3,4. Finally, some studies have shown that intrathecal or spinal administration of dexmedetomidine enhances anaesthetic effects5.
Intrathecal dexmedetomidine at doses of 5-10 µg has been shown to increase sensory blockade and improve postoperative analgesia when used in combination with local anaesthetics6. Dexmedetomidine also produces analgesia by depressing C-fibre transmitter release and by hyperpolarization of dorsal horn postsynaptic neurons7.
The use of intravenous dexmedetomidine in elective caesarean section for sedation is unlikely to be harmful to the infant because of its transfer into breast milk8.
The aim of the study, therefore, is to evaluate the effect of intrathecal dexmedetomidine in patients undergoing caesarean section under regional spinal anaesthesia and to observe the side effects of the drug.
This is a parallel, randomised, single-blind clinical trial. Implemented and approved in the obstetrics service of the Hospital Obrero N°2, a sample of 123 patients scheduled for caesarean section was taken from the finite population. The inclusion criteria were: age between 20 and 35 years, gestational age between 37 and 40 weeks, ASA (American Society of Anesthesiology) physical status classification I and II, no contraindication to regional anaesthesia and confirmation of participation in the study. On the other hand, age younger than 19 years and older than 36 years, presence of ASA III-IV, gestational age younger than 36 weeks and older than 41 weeks, twin pregnancy, refusal of spinal anaesthesia, hypertensive disorders of pregnancy, non-reassuring fetal status and refusal to participate were excluded from the study.
The 123 patients were randomly divided into three groups, with each group consisting of 41 patients. (see graph 1). The randomisation used a sequentially numbered table which was assigned to each group accordingly. Patients were unaware of which group they were assigned to. Within the allocation concealment, an anaesthesiologist was in charge of verifying the selection and assigning patients to each group. The investigation was terminated upon completion of the study groups.
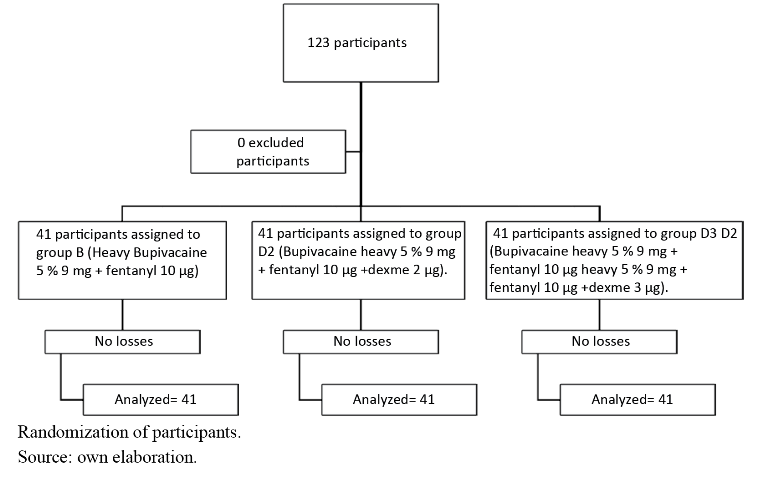
Group B (Bupivacaine heavy 5% 9 mg plus fentanyl 10 µg)
Group D2 (Bupivacaine heavy 5% 9 mg plus fentanyl 10 µg and dexmedetomidine 2 µg).
Group D3 (Bupivacaine heavy 5% 9 mg plus fentanyl 10 µg and dexmedetomidine 3 µg).
own elaborationProcedure
The patient was admitted to the operating theatre, where peripheral venous line patency was checked (branula No. 18 gauge), followed by non-invasive monitoring including pulse oximetry, three-lead electrocardiogram, heart rate, non-invasive blood pressure, and baseline parameters were recorded. At the same time, verification of the safe surgery checklist was performed.
Intravenous fluid infusion of Ringer Lactate solution at 10-12 ml/kg/h was performed.
Patient in sitting position, proceeded to spinal block with Whitacre N°25 needle, doses were administered according to the study groups. The most frequent puncture site was L4-L5 followed by L3-L4.
Subsequently, patient in dorsal decubitus with uterus lateralized to the left. Vital signs were monitored and recorded before the block, during the procedure, after the block, before obtaining the newborn, after obtaining the newborn, after the administration of oxytocics, every 15 min until the end of the procedure.
Hypotension was treated with etilefrine 2 mg, when systolic blood pressure is below 90 mmhg or presents a decrease of 15 % relative to baseline in mean arterial pressure. The presence of bradycardia was treated with atropine 0.5 mg EV, when it was below 50 beats per minute. Nasal prongs were placed if oxygen saturation was below 90 %.
The level of sensory blockade was assessed with an alcohol swab and the duration of motor blockade using the Bromage scale (regression of motor blockade to the ability to lift the extended leg - Bromage 0).
All side effects such as hypotension, bradycardia, nausea, vomiting, pruritus, tremor and respiratory depression were recorded.
Statistical analysis
Data were collected in Excel and analysed in SSPS version 25.0. Student’s t-test for continuous variables and the use of Chi-Square (χ²) test for nominal variables were compared. Confidence level of 95% and sampling error of 13%.
Mean and standard deviation were used for continuous variables while frequency was used for nominal variables.
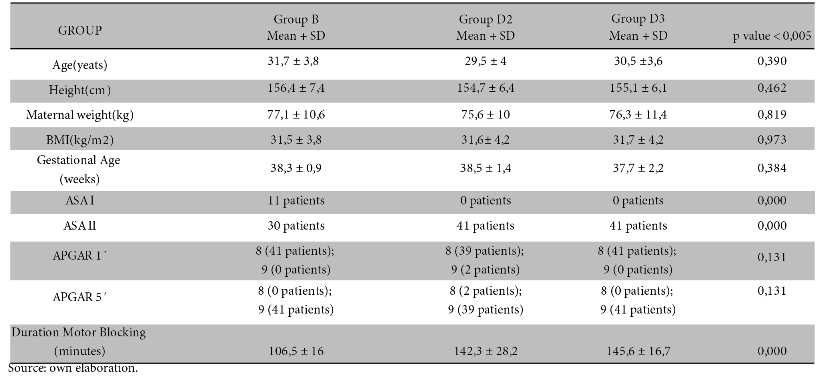
A total of 123 patients were studied, in each group 41 patients. The mean age in group B was 31.7 ± 3.8 years old; group D2 was 29.5 ± 4 years old; group D3 was 30.5 ± 3.6 years old. It was not significant.
As for gestational age, Body Mass Index (BMI) and APGAR of the newborn there is no statistically significant difference.
The most common ASA classification was ASA II with 30, 41 and 41 patients in group B, D2 and D3 respectively.
For the duration of the motor block it was 106.5 ± 16 minutes in group B; 142.3 ± 28.2 minutes in group D2 and 145.6 ± 16.7 minutes in group D3. It was significant between the study groups (p-value < 0.000). See table 1
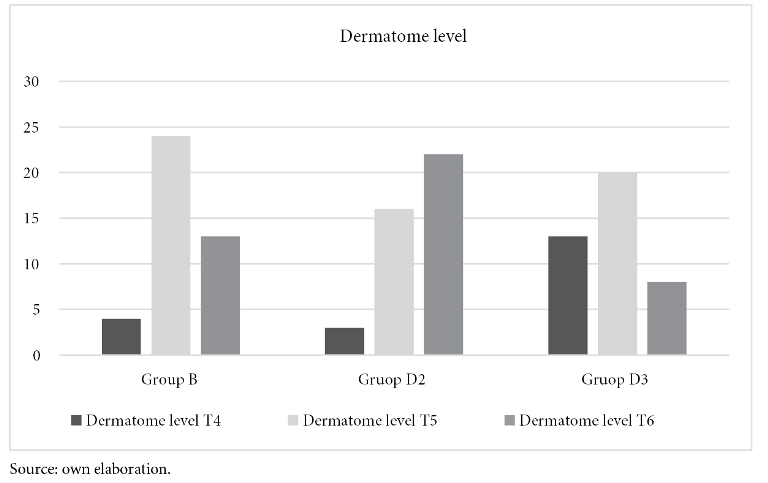
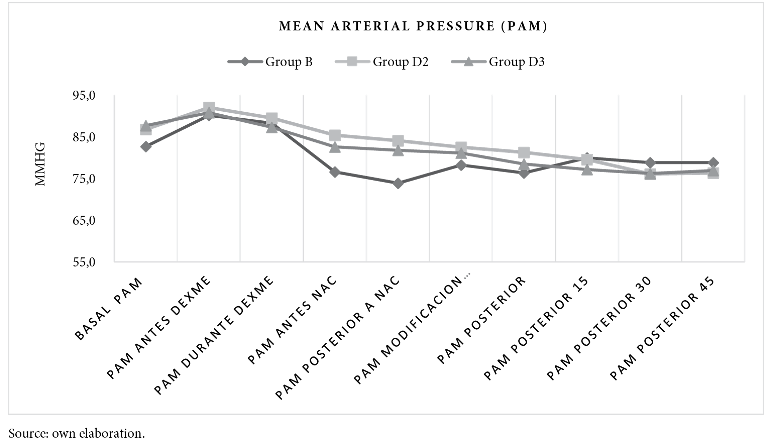
Whereas, mean arterial pressure measured before and after birth is statistically significant between groups B and D2 (p-value < 0.000), similar result between group B and D3 (p-value < 0.004). There is no significance between groups D2 and D3. See figure 2 and table 2.
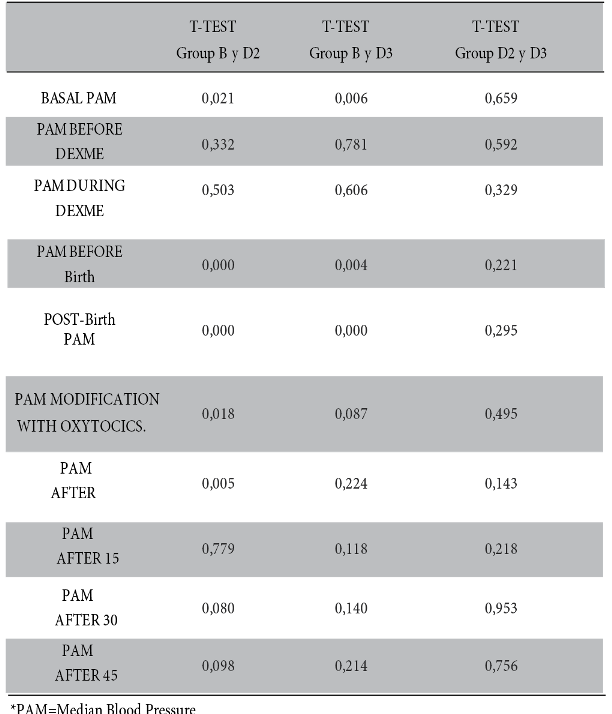
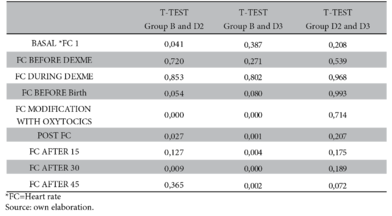
With regard to heart rate, there is a significant difference after administration of oxytocics (p-value < 0.000) between group B and D2 while in group B and D3 a higher significance is demonstrated after the use of oxytocics; at 15, 30 and 45 minutes after administration of local anaesthetics (p-value < 0.000, 0.001, 0.004, 0.000 and 0.002 respectively). On the other hand, there is no significance between groups D2 and D3 (Figure 3 and Table 3).

Ambient oxygen saturation remained between 93% and 96%. No statistical significance.
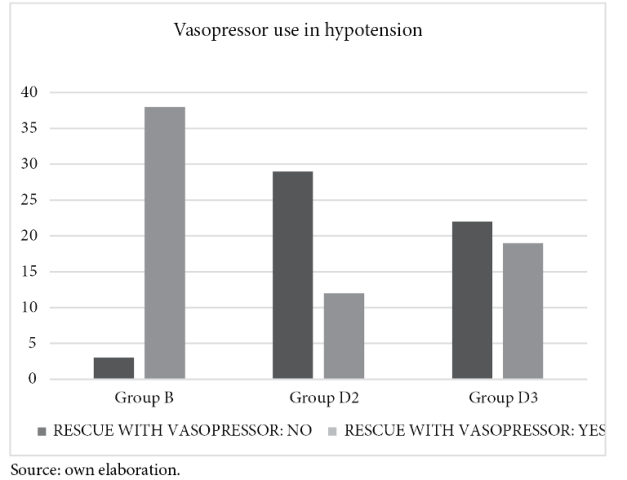
The use of vasopressors after hypotension was more frequent in group B in 38 patients, followed by group D3 in 19 patients and finally, in group D2 in only 12 patients. This was statistically significant with a Chi χ2 0.000 (figure 4).
volume of vasopressor (bolus etilefrin) used in group B was 3.44 ± 1.8 ml; for group D3 it was 1.8 ± 2.7 ml and for group D2 it was 0.85 ± 1.6 ml. Statistically significant between group B and D2; group B and D3. Not significant between group D2 and D3 (Table 4).
The total volume of vasopressor (bolus etilefrin) used in group B was 3.44 ± 1.8 ml; for group D3 it was 1.8 ± 2.7 ml and for group D2 it was 0.85 ± 1.6 ml. Statistically significant between group B and D2; group B and D3. Not significant between group D2 and D3 (Table 4).

Finally, the most frequent complication occurred in group D3, as follows: 7 patients presented nausea; only 3 patients presented vomiting and one patient presented pruritus; on the other hand, patients were under conscious sedation in group D2 and D3. The complication in group B was hypotension.
The study evaluates the effects of the use of intrathecal dexmedetomidine in patients undergoing caesarean section, which is little known and little used in our environment. Currently widely used worldwide, either intravenous, intranasal, spinal, epidural and other routes of administration.
Firstly, the mean age of the three study groups was 31, 29 and 30 years (group B, group D2 and group D3 respectively), similar to the study by Teymourian et al.9, where he reports 31 years for the control group and 30 years for the Dexmedetomidine group.
The physical status classification (ASA) in the study is reported to be frequently ASA II, as opposed to the report of Sun et al.10 where ASA I was found to be frequent.
To continue, an APGAR of 8 at one minute and APGAR 9 within 5 minutes was reported in all three study groups. Sun et al10 report APGAR of 7 and 10 at 1 minute and 5 minutes and mention the advantage of dexmedetomidine over other adjuvants. However, Nair et al.11 mention that dexmedetomidine has a high placental extraction, it is not transferred to the newborn. For Wan et al.4 the placental transfer rate is 0.68 in epidural anaesthesia.
Regarding the duration of the motor block, it was more relevant in group D3, although not significant compared to group D2. However, the prolongation of the motor block was significant in group B compared to group D3 - D2. Similarly, Li et al12 reported longer recovery time from motor block in the group using intrathecal dexmedetomidine at a dose of 10 µg, similar to the report of Wu et al13 in a systemic review.
While Qi et al14 in their study found longer regression time of motor block, but 2 ml bupivacaine plus 5 µg dexmedetomidine was administered. Ozdamar et al.7report that administration of 10 µg intrathecal dexmedetomidine without local anaesthetics in rats does not produce motor block or neurological neurotoxicity.
To mention the haemodynamic changes, mean arterial pressure was found to be more stable in the D2 group with fewer episodes of hypotension with no haemodynamic repercussions affecting the mother-child binomial without statistical significance against the D3 group.
In contrast, Bi et al15 found that the addition of 3 µg dexmedetomidine exerted better haemodynamic stability. Also, Abdallah et al16 report that haemodynamic side effects after spinal blockade were similar in the study groups (administration of 3 µg-10 µg dexmedetomidine plus bupivacaine or ropivacaine). But for Halder et al.17 they report no haemodynamic effects with the addition of 3 µg-5 µg dexmedetomidine.
With reference to heart rate, bradycardia was not reported in any study group, although in group B there was a decrease of 6 beats compared to the other study groups. Statistical significance was found between groups B and D3. Similarly, Patro et al.5 report mean heart rates between 66 and 76 beats per minute, hence statistical significance in both study groups (bupivacaine plus saline and dexmedetomidine 5 µg plus bupivacaine group).
The incidence of hypotension occurred in 38 patients in group B followed by group D3 in 19 patients. In a systemic review by Miao et al18 reported from 320 patients that the risk of hypotension was similar in both study groups with 95% CI 0.84, 1.42. For Naithani et al119 as a result of their study, and similarly Qi et al14 mentioned the incidence of hypotension is higher when higher doses of 5 µg dexmedetomidine are used. Sun et al10 found hypotension in all study groups without statistical significance.
The vasopressor (etilefrin 2mg bolus) used as rescue for hypotension management, in group B the total volume was 3.44 ± 1.8 ml, being higher than in group D2 and D3. Alegre et al.20 treated hypotension in the same way in one of the study groups, using etilefrin 2mg bolus with a total volume of 4.1 ± 0.76 ml in episodes of maternal hypotension.
With regard to complications, it was found that the D3 group presented more incidents of nausea and vomiting contrary to the report of Bao et al.3 which indicates a decrease in cases of nausea, vomiting, bradycardia and tremors following the use of intravenous dexmedetomidine while Liu et al.21 plus Xia et al.22 indicated that no side effects were observed with the addition of 5 µg intrathecal dexmedetomidine.
Regarding the sedation produced by the use of intrathecal dexmedetomidine, more than 95 % of patients were found to be sedated in the D2 and D3 group, while Teymourian et al.9 achieved ideal sedation of patients in the dexmedetomidine plus bupivacaine group and less use of anxiolytics.
Finally, the use of 2 µg intrathecal dexmedetomidine maintains better haemodynamic stability, with fewer episodes of hypotension and thus reduced vasopressor use, almost no complications in terms of nausea and vomiting, and excellent sedation in patients.
Limitations of the study, quantification of dexmedetomidine in newborn blood is not analysed.
Conflict of interest: The author has no conflict of interest.
Sources of financing: independent.

Group B (Bupivacaine heavy 5% 9 mg plus fentanyl 10 µg)
Group D2 (Bupivacaine heavy 5% 9 mg plus fentanyl 10 µg and dexmedetomidine 2 µg).
Group D3 (Bupivacaine heavy 5% 9 mg plus fentanyl 10 µg and dexmedetomidine 3 µg).
own elaboration








