Use of convalescent plasma in COVID-19 patients
Uso de plasma convaleciente en pacientes con COVID-19
Gaceta Médica Boliviana, vol. 43, no. 1, 2020
Universidad Mayor de San Simón
Artículos de Revisión
Received: 30 April 2020
Accepted: 15 June 2020

Abstract: Coronavirus disease 2019 (COVID-19) represents one of the biggest pandemics facing the world and is causing a global health crisis. To date, there are no approved therapies or vaccines for this disease. Several therapeutic options are being studied and vaccines are being developed. Convalescent plasma is an interesting therapeutic option against COVID-19, as it has been used successfully in other viral outbreaks in the past. This therapy consists of collecting plasma from individuals recovered from viral disease who have developed antibodies. It is considered the only short-term strategy to confer immediate immunity to susceptible individuals. In this review, we address the possible mechanisms of action of convalescent plasma, give an overview of the first studies conducted on COVID-19, as well as the procedures necessary to implement this therapy.
Keywords: COVID-19, SARS-CoV-2, convalescent plasma.
Resumen: La enfermedad por coronavirus 2019 (COVID-19) representa una de las mayores pandemias a las que el mundo se enfrenta y está produciendo una crisis sanitaria global. Hasta la fecha no se disponen de terapias o vacunas aprobadas para esta enfermedad. Se están estudiando varias opciones terapéuticas y desarrollando vacunas. El plasma convaleciente constituye una opción terapéutica interesante contra COVID-19, ya que sutilizó con éxito en otros brotes virales en el pasado. Esta terapia se consiste en la recolección de plasma de individuos recuperados de la enfermedad viral que desarrollaron anticuerpos. Es considerada la única estrategia a corto plazo, para conferir inmunidad inmediata a individuos susceptibles. En esta revisión se aborda los posibles mecanismos de acción del plasma convaleciente, damos una vista panorámica de los primeros estudios realizados en COVID-19, así como como los procedimientos necesarios para implementar esta terapia.
Palabras clave: COVID-19, SARS-CoV-2, plasma convaleciente.
In late December 2019, cases of unexplained pneumonia were reported in Wuhan, China. Testing concluded that the causative agent of these cases was a new coronavirus1. The World Health Organisation (WHO) has named the disease caused by this virus as COVID-19 (coronavirus disease 2019). The International Committee on Taxonomy of Viruses also named this new agent as Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)1.
As cases increased in the place of origin and surrounding areas, cases also began to be reported in other parts of the world. This led the WHO to initially declare COVID-19 a Public Health Emergency of International Concern and on 11 March it was officially declared a pandemic2. In Bolivia the first cases of COVID-19 were reported in March 2020 (departments of Oruro and Santa Cruz), currently cases are reported in all nine departments, with Santa Cruz being the most affected department3.
Transmission of SARS-CoV-2 is mainly by close contact, probably also by aerosols, but other routes of transmission are suggested and under study1. The clinical expression of COVID-19 is variable with the respiratory tract being the most affected site, but involvement of other organs is also possible4. The disease can be expressed as mild or even severe and life-threatening forms, the latter depending on certain risk factors4.
To date, there are no specific treatments or vaccines for this disease, making it an area of intense research5. Treatment is generally symptomatic as well as the respective management of complications.
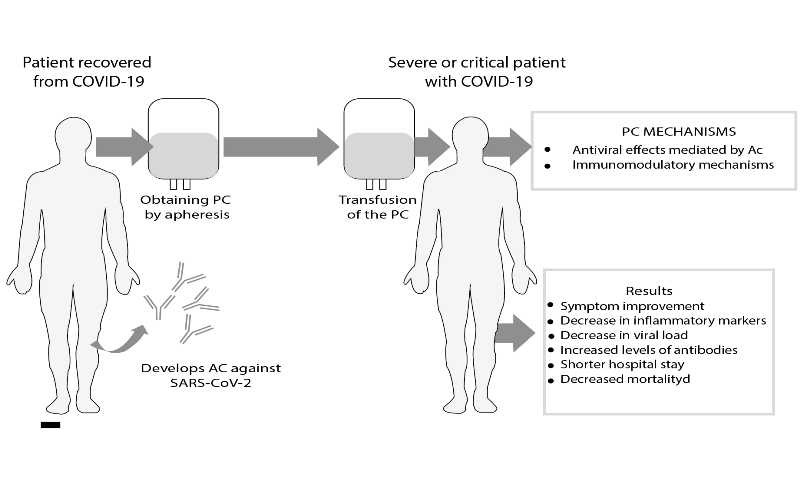
In the current scenario and with the steady increase of cases worldwide, urgent, feasible and effective treatment strategies are needed to deal with the disease, especially in severe cases. Recently, the use of convalescent plasma (PC) from recovered COVID-19 patients has been suggested as a treatment strategy in some cases, as in previous epidemics caused by other viral agents6. Initial results of this treatment in COVID-19 patients showed effectiveness in terms of clinical improvement, reduced viral load, fewer complications, as well as reduced mortality7,8.
Treatment with PC is available in many settings around the world and is relatively safe in terms of side effects. Considering that there are still no approved drugs for this disease, it could become a therapeutic strategy for some patients or be part of conventional treatment. Along these lines, in Bolivia, through the National Blood Programme, the protocol for treatment with PC for cases of COVID-19 began in May 20209.
This review describes the general aspects of PC treatment in COVID-19, underlying mechanisms, outcomes, as well as procedures necessary to implement this therapeutic strategy.
Historical Background
The use of convalescent blood products dates back to the late 1800s10. One of the first diseases in which their effectiveness was demonstrated was Spanish influenza (1918-1920)10. According to later analyses, convalescent blood products reduced mortality by up to 21%6.
Subsequently, during the SARS-CoV-1 epidemic of 2003, PC was used in several patients. The treatment resulted in lower mortality and shorter hospital stay6,11.
During the 2009 Influenza A H1N1 pandemic, PC was also used in patients with severe disease, demonstrating a favourable impact on mortality and decreased viral load6 . Furthermore, this treatment modality was well tolerated and no related complications were reported6.
In 2014, the WHO recommended empirical treatment with PC during Ebola outbreaks and more recently it was also used in patients with Middle East Respiratory Syndrome (MERS-CoV)6, 11.
Rationale and mechanism of action
To better understand the rationale for this treatment modality, we must first understand what we mean by PC. Zhang et al.11 consider PC as that which was collected from a patient who survived an infectious disease and therefore developed antibodies (Ac).
In this sense, PC treatment is a form of short-term passive immunisation, which allows the transfer of neutralising antibodies (NAbs) against a specific pathogen to a patient with the infection. These antibodies have the ability to neutralise the pathogen and thereby reduce deleterious pathophysiological events in the patient12.
In most viral diseases the peak of viraemia is reached before the patient develops sufficient Ac, with the consequence that certain susceptible patients develop major complications such as in SARS-CoV-26 infection. In this sense, the introduction of specific Ac contained in the PC may improve patient outcomes.
The mechanisms discussed above explain the antiviral effect of PC. However, other mechanisms attributable to treatment have been suggested. Recently, Rojas et al.13 conducted a review to explain the possible mechanisms of PC in COVID-19. They suggest that another important effect is imnunomodulation, which is produced by several mechanisms that are discussed below13:
F (ab’) 2 mechanisms suggest that PC may inhibit certain autoantibodies, which could play a role in COVID-19 and could be responsible for some complications such as thrombosis mediated by antiphospholipid antibodies. This mechanism could also interfere with the complement cascade, which is also implicated in the pathophysiology of the disease.
Mechanisms in Fc (crystallisable fragment), suggest that PC may produce saturation of FcRn (receptor for neonatal Fc), which would have effects on certain antibodies that could play a role in pathogenesis. Fcγ receptors are also important in the inhibition and modulation of immune cells, and mechanisms at this level are also proposed.
Effects on dendritic cells suggest that PC may enhance the anti-inflammatory properties of these cells, considering that the inflammatory response plays an important role in this disease.
Effects on T and B lymphocytes suggest that PC may modulate the balance and survival of different T lymphocyte populations. They also suggest effects at the level of B lymphocytes.
Effects on other cells suggest that PC may prevent migration of immune cells into lung tissue. From the above, PC would exert antiviral effects through specific NAbs, but also highlight the possible immunomodulatory mechanisms described at different levels, which could interfere with the pathophysiology of COVID-19 and therefore prevent the development of complications.
First studies of the use of convalescent plasma in COVID-19
The first reports on the use of PC in patients with COVID-19 are mostly case series, however, they are very valuable studies, since they provide the first experiences of the use of this treatment modality applied to this disease, and they are also the starting point for the development of current protocols.
Considering their importance, when commenting on each of these studies, some methodological aspects and the most noteworthy results will be described.
One of the first reports is by Shen et al7. In their study they describe the treatment with PC applied to five patients with critical COVID-19 in a hospital in Shenzhen, China. The selected donors were asymptomatic for at least ten days, tested negative for SARS-CoV-2 (by RT-PCR), had SARS-CoV-2 specific antibody titres of 1:1000 (by ELISA) and an NAbs titre greater than 40. Patients received two PC transfusions with a volume of 200-250 ml.
The authors highlight the improvement of several clinical-laboratorial parameters including SOFA (Sequential Organ Failure Assessment) score; arterial oxygen pressure/inspiratory oxygen fraction ratio (PaO2/FiO2), with substantial improvement after 12 days; normalisation of temperature; decrease of inflammatory markers (CRP, IL-6, procalcitonin); reduction of viral load; increase of IgG and IgM antibody titres and NAbs7. They also describe the gradual resolution of lung lesions on imaging studies, which in some patients was already evident on the third day after treatment7.
Duan et al.8 reported a series of ten patients with COVID-19 with severe disease criteria treated with PC in Wuhan, China. Seven of them tested positive for SARS-CoV-2 prior to transfusion. The selected donors were afebrile for more than 3 days, without any respiratory symptoms, and had 2 negative tests for SARS-CoV-2 by real-time polymerase chain reaction (RT-PCR) with 1-day interval. Patients received a transfusion of 200 ml of inactivated PC with neutralisation activity > 1: 640.
The treatment resulted in improvement of several clinical-laboratorial parameters: symptoms improved within 1-3 days; there was remission of lymphopenia; reduction of inflammatory marker levels; improvement of liver function and remission of lung lesions on imaging studies8. In addition, an increase in NAbs titres was observed in five patients, and in the seven patients with a previous positive test for SARS-CoV-2, negativisation was observed8 .
Another study, also conducted in Wuhan, reported a series of six patients with COVID-19 in critical condition or with poor outcome despite standard treatment14. The selected donors were afebrile for at least 3 days, without any respiratory symptoms, with 2 consecutive negative tests for SARS-CoV-2 by RT-PCR and after 3 weeks from the onset of illness. Patients received transfusion of 200 ml PC, after which five patients showed resolution of lung lesions on imaging studies.
In Korea, two patients with COVID-19 with severity criteria, older than 60 years with acute respiratory distress syndrome (ARDS)15 were reported. The patients received two PC transfusions (250 ml) every 12 hours. Both patients showed significant improvement of clinical-laboratory parameters as well as independence from mechanical ventilation. The SARS-CoV-2 test was negative in both patients post-transfusion (day 20 and 26)15.
Zeng et al.16 described the outcomes of twenty-one patients older than 60 years with COVID-19 who were admitted to the intensive care unit. Six of the patients were treated with PC and the rest were considered as a control group. This study not only assessed treatment outcomes, but also viral clearance.
Mortality in the treatment group was 5/6 and in the control group it was 14/15. 100% of patients treated with PC had undetectable SARS-CoV-2 compared to 21.4% in the control group (p = 0.005), and a longer survival time was evident in the treated group16. This study denotes the effectiveness of PC in terms of viral clearance, however, as suggested by the authors, this treatment modality should be initiated before the patient becomes critically ill.
One aspect on which all the studies discussed agree is that the use of PC was limited to patients with severe or critical COVID-19 and in some cases it was the therapeutic strategy of choice in the absence of clinical improvement. It should be noted that almost all studies reported favourable results. Furthermore, the treatment was generally well tolerated and no significant adverse reactions were reported in any of the studies.
Regulation
El Treatment with PC in patients with COVID-19 is an emerging therapeutic strategy in the current pandemic. In this regard, we note that at the time of writing, it is not yet an approved treatment by the relevant regulatory bodies, due to the lack of clinical trials.
In the United States, the Food and Drug Administration (FDA) authorised the use of PC for COVID-19 as an investigational product through three routes: clinical trials, expanded access and as an emergency investigational new drug (IND) in a single patient 17 .
Furthermore, a publication by the International Society of Blood Transfusion (ISBT)18suggests that the clinical use of PC should be handled as an experimental therapy and therefore meet all ethical and legal standards. This body also recommends that, given the experimental nature, donor and patient samples should be stored for further studies.
In Bolivia, consistent with the aforementioned regulations, treatment with PC for COVID-19 was authorised as an experimental treatment subject to safety and efficacy evaluation, being regulated by the National Blood Programme9.
Donor selection
Donor selection is the key step in initiating a PC treatment protocol for COVID-19. This requires national or hospital registries and the necessary public awareness to promote donation.
One of the problems faced in various parts of the world, especially during the reporting of the first cases, is the lack of recovered patients. Another problem that arises is the lack of interest in donation, from our point of view this is a very common problem in our country. We stress that donation of PC by a recovered patient should be a voluntary and altruistic act9 .
The selection criteria for PC donors in the case of COVID-19 were recently defined based on the studies developed to date and according to the corresponding institutions (Table 1)18,19. As for a standard donation, a clinical evaluation of the donor must be carried out, and it is also suggested that data related to the diagnosis and course of the disease be recorded for subsequent studies18.

PC collection and processing
According to current recommendations, PC collection should only be performed by apheresis, and the use of whole blood as a source of PC is not recommended18. The apheresis procedure should be performed according to standard protocol, in certified health facilities and by qualified personnel 9,18,19. The volume to be collected should be in relation to the donor’s parameters; it is suggested that a volume of 200-600 ml of plasma be collected18.
Once the PC has been obtained, it should undergo a pathogen reduction process18.When feasible, total titres of Ac and NAbs should be determined18, 19 NAbs are considered to correlate best with efficacy, although this test is not readily available in many settings20.
Interestingly, antibody titres in convalescent patients are related to the initial viral load. Considering this aspect, it has been suggested that patients who had more symptomatology develop higher antibody titres19. Further studies are needed to take this consideration into account during donor selection.
Zhang et al.21evaluated Ac levels in six donors recovered from COVID-19, five of whom had high Ac titres and were eligible for the criteria used in their study. This suggests that most donors meeting the established selection criteria would probably have high titres of Ac. We note, however, that to date there is no recommended optimal level of Ac in PC to ensure efficacy.
In relation to probable viraemia in PC, according to the suggested criteria for donor selection, this would not exist; however, the detection of SARS-CoV-2 in plasma collected prior to transfusion is suggested19.
As previously mentioned, as this is an experimental treatment, donor serum samples should be stored for future research.
The collected PC should be divided into aliquots of 200-250 ml and properly labelled, specifying the name of the product and that it is an experimental treatment9,18. The unit can be used immediately or cryopreserved according to standard norms18 . The interval between apheresis donations is generally 2 weeks and no less than 7 days18,19
Patient selection, administration and dosage
There are currently no recommendations for the selection of patients with COVID-19 as candidates for treatment with PC. In the studies conducted to date, treatment has mainly been performed in patients with severe or critical illness.
The FDA, to facilitate IND application, suggests following the National Protocol for Expanded Access to Treatment eligibility criteria, which are as follows17:
Severe illness is defined as one or more of the following: respiratory distress (dyspnoea); respiratory rate ≥ 30/min; oxygen saturation ≤ 93%; PaO2/ FiO2 <300; pulmonary infiltrates > 50% in 24-48 hours.
Life-threatening illness is defined as one or more of the following: respiratory failure; septic shock; multiple organ dysfunction or failure.
In Bolivia, following the above guidelines, treatment for severe and critical cases of COVID-199 is proposed.
There is still insufficient evidence to support the use of PC in earlier phases, however, considering the mechanisms of action and the pathophysiology of the disease, we could assume greater benefits if treatment is instituted in earlier phases. Zeng et al.16 also suggested that treatment might be more beneficial in earlier stages of the disease, after showing significant viral clearance after treatment, but despite this finding and because the patients were in very advanced stages, mortality was high. Further studies are needed to define the optimal stage to indicate PC treatment in patients with COVID-19.
The transfusion process is the same as with fresh frozen plasma (FFP), respecting ABO compatibility and according to nationally established standards9 . Regarding dosage, recommendations suggest administering 200 to 400 ml as a single dose and one or two additional doses depending on the severity and tolerance of the patient7,18. The administration time, as with FFP, is 10-20 ml/kg/hour22; a longer administration time should be considered in patients at risk of circulatory overload.
Adverse reactions
Como As with any blood component, PC transfusion carries the risk of adverse reactions such as: non-hemolytic febrile reactions, allergic reactions, transfusion-associated infections, haemolytic reactions and more severe reactions such as transfusion-related acute lung injury (TRALI) or transfusion-associated circulatory overload (TACO)23.
We emphasise TRALI and TACO as they are the most severe and potentially life-threatening adverse reactions24. Several risk factors for these complications have been described, including mechanical ventilation, positive water balance, shock, advanced age, cardiac dysfunction, among others23,24. Several of the above factors may be present in patients with severe or critical COVID-19.
Sufficient attention must be paid to identify these complications in a timely manner. While in most severe and critically ill COVID-19 patients, it may be difficult to identify these complications, it is important to be aware of these complications, any sudden deterioration of respiratory parameters or haemodynamic instability following transfusion should be suspected. Both TRALI and TACO usually present within 6 hours after transfusion24.
In the current scenario within a PC protocol for COVID-19, the donor should preferably be male or a woman with no history of pregnancy or miscarriage, which is a factor that decreases the likelihood of TRALI9. In the case of TACO, we suggest identifying risk factors and considering a longer infusion time with strict control of water balance.
In relation to the risk of SARS-CoV-2 transmission, it is not considered a relevant agent of transmission through transfusions; only 1% of symptomatic patients would have the virus RNA in their blood20. As previously indicated, some recommendations suggest the detection of virus RNA in plasma collected prior to transfusion18.
A recent study evaluated 5000 hospitalised patients with severe or life-threatening COVID-19 who received PC25. The incidence of all serious adverse events in the first four hours after transfusion was less than 1% and a reported mortality rate of 0.3%. Of thirty-six serious adverse events reported, only two were considered to be directly related to the transfusion.
Consequently, while there is a risk of adverse reactions associated with PC transfusion in patients with COVID-19, the studies available to date conclude that the incidence is very low.
Conclusions
COVID-19 represents one of the greatest challenges to medicine in recent years and probably the biggest global health crisis. Disease-specific treatments and vaccines are not yet available.
PC is a therapeutic strategy that has been used in other viral diseases in the past with positive results. Early studies evaluating this treatment in patients with especially critical COVID-19 show that it has a positive impact on patient recovery; reduction of viral load and reduction of mortality. Furthermore, in general no significant adverse effects were reported.
Treatment with PC is a therapeutic measure that, in the face of the emergence of a new viral agent such as SARS-CoV-2, can become a readily available treatment strategy.
We recognise that a definitive conclusion on the effectiveness, optimal doses and ideal timing of PC treatment in patients with COVID 19 cannot yet be reached. Results from randomised clinical trials, many of which are ongoing, are urgently required.
Bibliographic references
1. Wang LS, Wang YR, Ye DW, Liu QQ. A review of the 2019 Novel Coronavirus (COVID-19) based on current evidence. Int J Antimicrob Agents 2020:105948
2. Cucinotta D, Vanelli M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020;91(1):157-160.
3. Ministerio de Salud. Guía para el manejo del COVID-19. Versión mayo de 2020. La Paz: Unidad de epidemiologia DGSS; 2020
4. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020; 382(18):1708-1720.
5. Rismanbaf A. Potential Treatments for COVID-19; a Narrative Literature Review. Arch Acad Emerg Med. 2020;8(1):e29.
6. Chen L, Xiong J, Bao L, Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis. 2020;20(4):398-400.
7. Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, et al. Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma. JAMA. 2020;323(16):1582-15890.
8. Duan K, Liu B, Li C, Zhang H, Yu T, Qu J et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci. 2020;117(17):9490-9496.
9. Programa Nacional de Sangre del Ministerio de Salud. Obtención y uso del plasma hiperinmune obtenido por plasmaferesis en pacientes con COVID-19. Version mayo 2020. La Paz: Programa Nacional de Sangre – DGSS; 2020
10. Brown BL, McCullough J. Treatment for emerging viruses: Convalescent plasma and COVID-19. Transfus Apher Sci. 2020;102790.
11. Zhang J, Xie B, Hashimoto K. Current status of potential therapeutic candidates for the COVID-19 crisis. Brain Behav Immun. 2020;S0889-1591(20)30589-4.
12. Alzoughool F, Alanagreh L. Coronavirus drugs: Using plasma from recovered patients as a treatment for COVID-19. Int J Risk Saf Med. 2020;31(2):47-51.
13. Rojas M, Rodríguez Y, Monsalve DM, Acosta-Ampudia Y, Camacho B, Gallo JE, Rojas-Villarraga A, et al. Convalescent plasma in Covid-19: Possible mechanisms of action. Autoimmun Rev. 2020:102554.
14. Ye M, Fu D, Ren Y, Wang F, Wang D, Zhang F, et al. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China. J Med Virol [Internet] 2020 [citado el 20 de mayo de 2020];10.1002/jmv.25882. Disponible en: https://onlinelibrary.wiley.com/doi/full/10.1002/jmv.25882
15. Ahn JY, Sohn Y, Lee SH, Cho Y, Hyun JH, Baek YJ, et al. Use of Convalescent Plasma Therapy in Two COVID-19 Patients with Acute Respiratory Distress Syndrome in Korea. J Korean Med Sci. 2020;35(14):e149.
16. Zeng QL, Yu ZJ, Gou JJ, Li GM, Ma SH, Zhang GF, et al. Effect of Convalescent Plasma Therapy on Viral Shedding and Survival in COVID-19 Patients. J Infect Dis. 2020; 222 (1):38-43.
17. Food and Drug Administration. Recommendations for Investigational COVID-19 Convalescent Plasma [Internet]. FDA. 2020 [citado el 2 de mayo de 2020]. Disponible en: https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/recommendations-investigational-covid-19-convalescent-plasma.
18. Epstein J, Burnouf T. Points to consider in the preparation and transfusion of COVID-19 convalescent plasma. Vox Sang [Internet] 2020 [citado el 25 de mayo de 2020];10.1111/vox.12939. Disponible en: https://onlinelibrary.wiley.com/doi/full/10.1111/vox.12939
19. Tiberghien P, de Lamballerie X, Morel P, Gallian P, Lacombe K, Yazdanpanah Y. Collecting and evaluating convalescent plasma for COVID-19 treatment: why and how?. Vox Sang [Internet] 2020 [citado el 25 de mayo de 2020];10.1111/vox.12926. Disponible en: https://onlinelibrary.wiley.com/doi/full/10.1111/vox.12926
20. Bloch EM, Shoham S, Casadevall A, et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest. 2020;130(6):2757-2765.
21. Zhang L, Pang R, Xue X, Bao J, Ye S, Dai Y, et al. Anti-SARS-CoV-2 virus antibody levels in convalescent plasma of six donors who have recovered from COVID-19. Aging. 2020;12(8):6536-6542.
22. Robinson S, Harris A, Atkinson S, Atterbury C, Bolton-Maggs P, Elliott C et al. The administration of blood components: a British Society for Haematology Guideline. Transfus Med. 2018;28(1):3-21.
23. Pandey S, Vyas GN. Adverse effects of plasma transfusion. Transfusion. 2012;52 Suppl 1(Suppl 1):65S-79S.
24. Delaney M, Wendel S, Bercovitz RS, Cid J, Cohn C, Dunbar NM, et al. Transfusion Reactions: Prevention, Diagnosis, and Treatment. Lancet. 2016;388(10061):2825-2836.
25. Joyner JM, Wright RS, Fairweather D, Senefeld JW, Senefeld J, Bruno K, Klassen S, et al. Early safety indicators of COVID-19 convalescent plasma in 5,000 patients. medRxiv [Internet]. 2020 [citado el 20 mayo de 2020]. Disponible en: https://www.medrxiv.org/content/10.1101/2020.05.12.20099879v1.
Author notes
E-mail: nelson_ninag@outlook.com
Alternative link
http://www.scielo.org.bo/scielo.php?script=sci_arttext&pid=S1012-29662020000100013&lng=es&nrm=iso (html)

