Microvascular decompression for the treatment of trigeminal neuralgia
Descompresión microvascular para el tratamiento de la neuralgia del trigémino
Gaceta Médica Boliviana, vol. 43, no. 1, 2020
Universidad Mayor de San Simón
Artículos de Revisión
Received: 19 November 2019
Accepted: 23 February 2020

Abstract: Trigeminal neuralgia is defined as (paroxysmal, unilateral, severe, penetrating, short-lasting, recurrent pain in the distribution of one or more branches of the fifth cranial nerve). It may be essential or secondary. Its prevalence is high and has been increasing along with life expectancy; it constitutes 89% of facial neuralgias in people over 60 years of age. Diagnosis is based on the clinical picture with physical and neurological examination. There are complementary studies such as tomography and brain resonance, which are focused on differentiating between essential or secondary neuralgia. This pathology continues to be an unknown disease for many general practitioners and what is even worse, it is poorly managed by many of the specialists in charge of it. This paper summarises the main anatomic-clinical and pathophysiological features and a description of the surgical technique of microvascular decompression as the best therapeutic option for trigeminal neuralgia.
Keywords: microvascular decompression, gasser’s ganglion, trigeminal neuralgia.
Resumen: La neuralgia del trigémino se define como (dolor paroxístico, unilateral, severo, penetrante, de corta duración y recurrente en la distribución de una o varias de las ramas del V par craneal). Puede ser esencial o secundaria. Su prevalencia es alta y ha ido aumentando junto con la expectativa de vida, constituye el 89% de las neuralgias faciales en personas mayores de 60 años. Su diagnóstico se basa en el cuadro clínico con exploración física y neurológica. Existen estudios complementarios como tomografía y resonancia de encéfalo, los cuales están enfocados a diferenciar entre una neuralgia esencial o secundaria. Esta patología continúa siendo una enfermedad desconocida para muchos médicos generales y lo que resulta aún peor, mal manejada por muchos de los especialistas encargados de la misma. En este trabajo se resumen las principales características anatomocli4nicas, fisiopatológicas, y una descripción de la técnica quirúrgica de la descompresión microvascular como la mejor opción terapéutica para la neuralgia del trigémino.
Palabras clave: descompresión microvascular, Ganglio de Gasser, Neuralgia del trigémino.
Trigeminal neuralgia or tic douloureux is a paroxysmal, lancinating facial pain, described as an electric shock, lasting seconds (rarely up to one minute), often triggered by a sensory stimulus in specific areas of the face (the so-called trigger zones) and distributed over the innervation territory of one or more branches of the trigeminal nerve, without neurological deficit. The pain appears when eating, brushing teeth, touching the face, cold air. Characteristically, the painful discharge is not nocturnal since these trigger zones are not stimulated during sleep.
Rarely, it manifests as a status trigeminus, a rapid succession of tic-like spasms provoked by any stimulus1.
Literature review
Epidemiology
It is a disease that appears above the age of 50 years (average: 63 years). For some authors it is more frequent in men (1.2:1)2, while for others it is more frequent in women3. The annual incidence is estimated at 4 per 100,000 inhabitants. Exceptionally, it is familial. Most frequently, the pain affects the right hemiface (60% of cases); in 39% of cases it occurs on the left side; it is bilateral in 1%2. In cases of bilaterality, the pain usually occurs alternately. Multiple sclerosis is the most important predisposing factor for bilateral neuralgia, such that approximately 18% of patients with bilateral trigeminal neuralgia have multiple sclerosis1.
The trigeminal branches V2 and V3 together are the most frequently involved (42% of cases); then the 2nd branch alone (20%); in 17% of cases V3 is affected; in 14% of cases V1 together with V2; involvement of all three branches simultaneously occurs in 5%; the rarest distribution corresponds to V1 (2% of cases)2.
Trigeminal neuralgia can be primary (called idiopathic or essential by others) or secondary (also known as symptomatic). Primary trigeminal neuralgia is when no cause is found to explain the condition; these are the most common.
Secondary, when an underlying cause is discovered. The clinical manifestations are paraesthesias and dysesthesias, with pain becoming a secondary part of the picture. In addition, deficient signs are discovered in the neurological examination. They give a picture of atypical neuralgia4. Among the causes of secondary neuralgia are lesions of the cerebellopontine angle, conditions affecting the brainstem, various pathologies of Meckel’s cavum, tumours of the middle fossa, skull base metastases, acoustic neurinoma and pituitary adenomas, the latter being the most frequent of all.
When trigeminal neuralgia is caused by a brain tumour, it usually has atypical characteristics, the pain is usually constant, deficient signs are discovered on neurological examination, mainly loss of sensitivity (although in some patients the examination may be normal at first) and the painful picture appears in a relatively young subject. In addition, subjects with typical trigeminal neuralgia respond to carbamazepine initially, which is not the case if the facial pain is atypical5.
Anatomophysiological aspects
Los The sensory receptors of the trigeminal nerve pick up afferent stimuli from the skin, mucosa, muscle spindles and joints. These receptors are of the same type as those of the spinal nerves. They include nociceptors, which are structures specialised in the perception of nociceptive stimuli, consisting of finely myelinated type A axons with a high threshold for mechanical activation6.
The afferent fibres of the sensory receptors are grouped into three peripheral nerves, which are the ophthalmic (V1), maxillary (V2) and mandibular (V3) (Figure 1). The neuronal bodies of the trigeminal primary afferent fibres are contained in the semilunar ganglion or Gasser’s ganglion, which is located in an excavation of the apex of the pons surrounded by the meningeal coverings, forming the so-called cavum of Meckel (Figure 1). Behind the ganglion is the trigeminal cistern, which contains cerebrospinal fluid; this cistern communicates with the cisterns of the posterior fossa via the so-called porus trigemini. Gasser’s ganglion is somatotopically organised medially to laterally7, so that the neurons of the ophthalmic branch are located anteromedially, those of the mandibular branch posterolaterally and those of the maxillary branch in the middle of the other two. Although the ophthalmic and maxillary branches are well separated, there is some overlap between the maxillary and mandibular branches8.
The trigeminal root runs from Gasser’s ganglion to the pons; it consists of a major or sensory portion, a minor or motor portion and an intermediate or accessory portion8 (Figure 1).

1. Ganglion of Gasser.
2. Ophthalmic branch (V1)
3. Maxillary branch (V2).
4. Mandibular branch (V).
5. Optic nerve.
6. Optic tract
7. Thalamus.
8. Optical radiation.
9. Frontal horn of the lateral ventricle.
10. Corpus callosum
Pathophysiology
Trigeminal neuralgia is probably caused by an ephaptic or aberrant transmission, occurring within the same nerve, from larger diameter type A myelin fibres to type A delta and C (noncirrhotic) amyelin fibres1.
Pathogenesis may be due to:
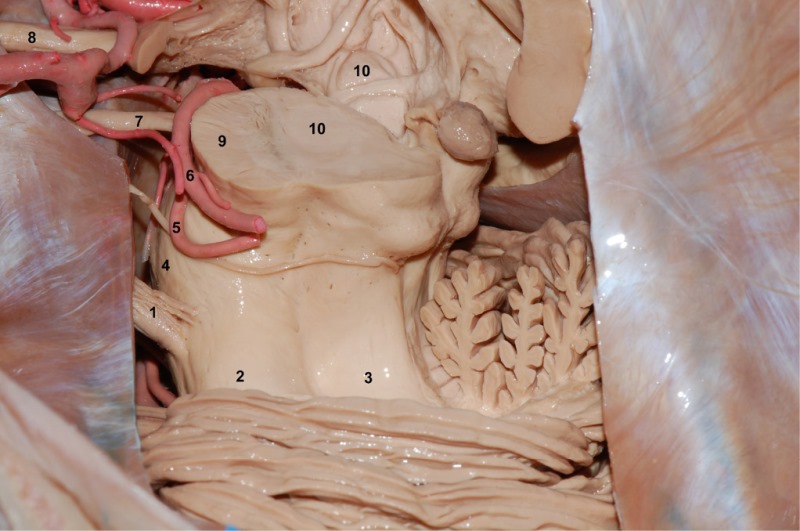
1. Trigeminal nerve.
2. Middle cerebellar peduncle.
3. Superior cerebellar peduncle.
4. Protrusion.
5. Superior cerebellar artery.
6. Posterior cerebral artery
7. Oculomotor nerve.
8. Optic nerve.
Clinical history and neurological examination
Medical history:
Accurate description of the location of the pain to determine which divisions of the trigeminal nerve need to be treated.
Determine the time of onset and triggering mechanisms.
Establish the presence of pain and the duration of asymptomatic intervals.
Determine what drug treatment the patient has tried, the duration, side effects, dosages and degree of response.
Investigate history of herpes vesicles.
Neurological examination:
The examination should be normal
Assess sensation in the 3 divisions of the trigeminal nerve bilaterally (include corneal reflexes).
Assess masseter function (chewing) and pterygoid function (with the mouth open, the chin deviates to the diseased side in case of paresis).
Assess external ocular motor function.
If the examination reveals any neurological deficit in a patient who has not previously undergone surgery, a structural cause should be suspected as the origin of the nerve pain (tumour, multiple sclerosis). Cranial magnetic resonance imaging is mandatory for any trigeminal neuralgia1.
Diagnostic studies
MRI (with FIESTA sequence) is often used to evaluate these patients for possible intracranial tumours or plaques of multiple sclerosis, especially in cases with atypical features1.
Differential diagnosis:
Treatment
1. Medical treatment
The drug of choice is carbamazepine: it produces complete or acceptable relief in 69% of cases. With prolonged use, the therapeutic response decreases by 50% despite a progressive increase in the dose administered. If 600 or 800 mg/day is tolerated and does not produce relief, a diagnosis other than trigeminal neuralgia should be suspected12.
Baclofen: Second choice. May be more effective if given with low doses of carbamazepine. Abrupt withdrawal should be avoided, otherwise it may cause hallucinations and seizures.
Gabapetin or pregabalin: An anticonvulsant that has some efficacy in trigeminal neuralgia1
Phenytoin: May be effective in intravenous form in patients who report severe pain that prevents them from opening their mouth for oral treatment.
Other drugs: Clonazepam: Clonazepam: Clonazepam is an anticonvulsant that is effective in trigeminal neuralgia. Other drugs: Clonazepam, useful in 25% of cases. Amitriptyline, for its analgesic properties against deaferentiation pain and its antidepressant properties.
2. Surgical treatment
Reserved for cases refractory to medical treatment, or when the side effects of medication exceed the risks and disadvantages of surgery1,13.
Surgical options
Avulsion of peripheral branches: This comprises a series of procedures consisting of blocking the peripheral branch affected by pain or blocking the trigger zones. It can be performed with alcohol, phenol or by neurectomy of the affected trigeminal branch. It produces pain relief lasting from a few months to 1 or 2 years4.
Trigeminal bulbar tractotomy: Currently no longer performed.
Percutaneous rhizotomy with glycerol: This consists of injecting a volume of 99.9% anhydrous glycerol into the cistern of Meckel’s cavum14. It provides symptomatic relief in 90% of patients with a low incidence of residual sensory defect. Its main drawback is its high recurrence rate, with less than 50% of treated patients enjoying good analgesia in the long term.
Differential radiofrequency thermocoagulation of Gasser’s ganglion: This is based on the initial hypothesis that the C (amyelinic) and thinly myelinated A (delta) fibres, which transmit pain sensitivity, must be more vulnerable to destruction by controlled thermal elevation (70°C) than the thickly myelinated fibres, which transmit proprioceptive and tactile sensitivity (A alpha and A beta, respectively). This selective destruction would therefore allow the preservation of tactile sensitivity and the disappearance of pain. Experimental studies have now shown that thermocoagulation affects practically all types of fibres equally.
Percutaneous compression of Gasser’s ganglion (Mullan-Lichtor): This procedure is an adaptation of the direct subtemporal trigeminal compression of Gasser’s ganglion performed by Shelden in 1955. This author noted that the common denominator of the decompressive techniques performed until then was precisely the mechanical injury exerted on the ganglion or the root while attempting to surgically release one of these two structures15. The percutaneous procedure as it is performed today was introduced by Mullan and Lichtor in 1978 and published by the same authors in 198316.
Surgical techniques on the trigeminal root (microvascular decompression, posterior trigeminal rhizotomy and Spiller-Frazier technique).
Microvascular decompression (MVD)
Se It is based on the theory that trigeminal neuralgia is due to compression of the posterior root by anomalous vascular elements17, mainly the superior cerebellar artery (80% of cases) and sometimes by the persistent primitive trigeminal artery. On the other hand, other authors have demonstrated vascular compression of the posterior root in up to 50% of autopsies of patients who did not have trigeminal neuralgia.
Technique: This consists of performing an asterional microcraniectomy. The aim is to separate the artery from the nerve root with a Teflon or similar patch as close to the brainstem as possible. This way of “injuring” or “deafferentiating” the root improves the pain, somewhat contradicting the pathophysiological hypothesis that trigeminal neuralgia corresponds to a form of pain due to deafferentiation.
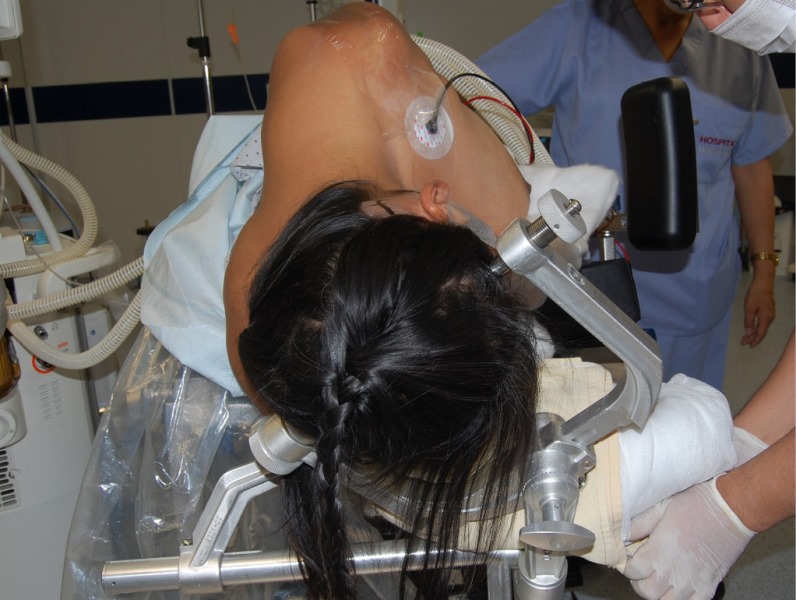
Positioning18.
Rotation: head parallel to the floor or rotated 10-15° for the contralateral side. (Figure 3).
Lateral tilt of the head, to separate the head from the shoulder and allow more and better mobilisation of the microscope.
Neck flexion: leave a space of 2 fingers between the chin and the sternum. 4. Upper shoulder retracted caudally with tape (Figure 3).
Surgical approach
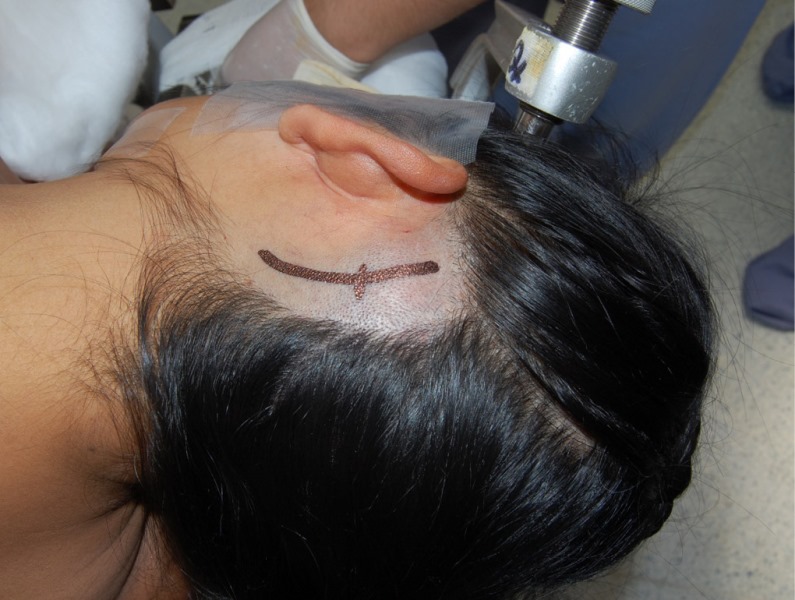
1. Skin incision18: vertical incision 3-5 cm long, 5 mm medial to the mastoid notch; in patients with a short neck the incision may be slightly longer. 75% of the incision is inferior to the transverse sinus, 25% superior (Figure 4).
2. Trepanation :
1 cm inferior and 1 cm medial to the asterion19.
If the asterion is not easily identified or if there are doubts about the reliability of the asterion as a reference point for the junction of the transverse and sigmoid sinuses, place the trephine directly over the mastoid emissary vein draining superolaterally into the sigmoid sinus.
3. Asterional microcraniectomy: the opening of the bone as close as possible to the transverse and sigmoid sinus. The position of the transverse sinus can be approximated by a line drawn from the posterior base of the zygomatic arch to the inion, or approximately 2 fingers width above the upper end of the mastoid notch.
The lateral limit of the open bone is the sigmoid sinus. And the upper limit is the transverse sinus.
The diameter of the craniectomy should be only 2 cm. Apply bone wax to block any possible opening to the mastoid air cells (Figure 5).
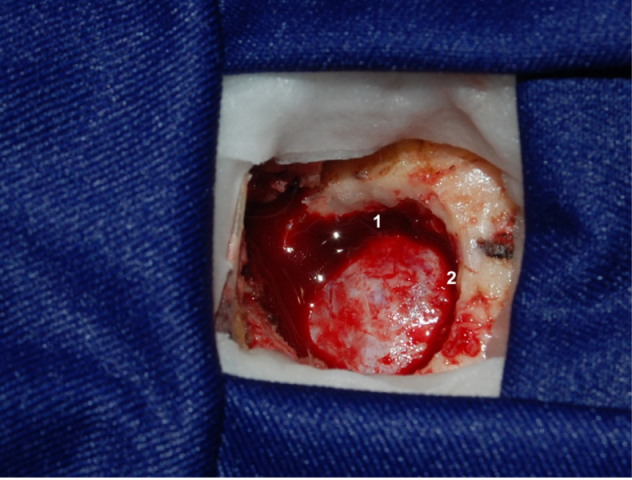
1. Sigmoid sinus.
2. Transverse sinus.
4. Dural opening: either a curved line parallel to the transverse and sigmoid sinus or an inverted “T”.
5. Minimal or no cerebellar retraction is usually required.
6. Allow cerebrospinal fluid in the cerebello-cerebellar angle to drain before proceeding.
7. Follow the junction of the tentorium with the petrous bone. It is essential to cut the arachnoid adhesions. After this manoeuvre, a retractor can be placed to displace the cerebellum medially.
8. Superior petrous vein: ideally it should be preserved. If it tears, it must be plugged or, if necessary, coagulated and sectioned.
9. The trigeminal nerve is deeper than the VII-VIII complex, so the latter cranial nerves should not be visible, if they are visible it is necessary to mobilise the spatula superiorly, as occasionally gentle traction can cause hearing loss.
Nerve decompression.
1. The arachnoid overlying the fifth nerve is tightly adherent at the cerebellopontine angle, so it is necessary to perform a sharp dissection, using microscissors (Figure 6 A). The trigeminal nerve and its relationship to the pons must be fully exposed.
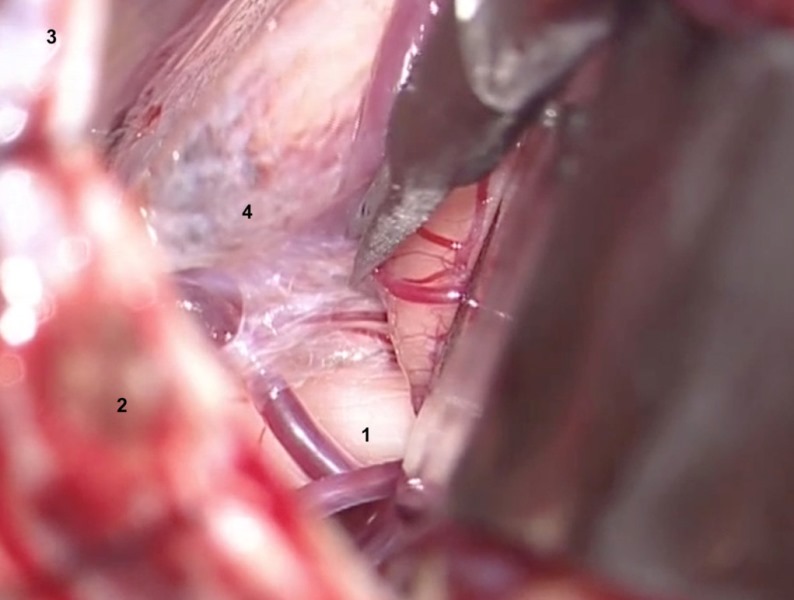
1. Trigeminal nerve.
2. Sigmoid sinus.
3. Transverse sinus.
4. Arachnoid.
2. The fifth nerve may be atrophic if percutaneous rhizotomies have been performed previously.
3. Arteries and/or veins compressing the V pair must be dissected from the nerve. The nerve must be inspected and freed from the arterial vessels from its origin in the brainstem to its entry into the cavum of Meckel18. The veins can be coagulated and then divided (to avoid recanalisation) (Figures 6 B and C).
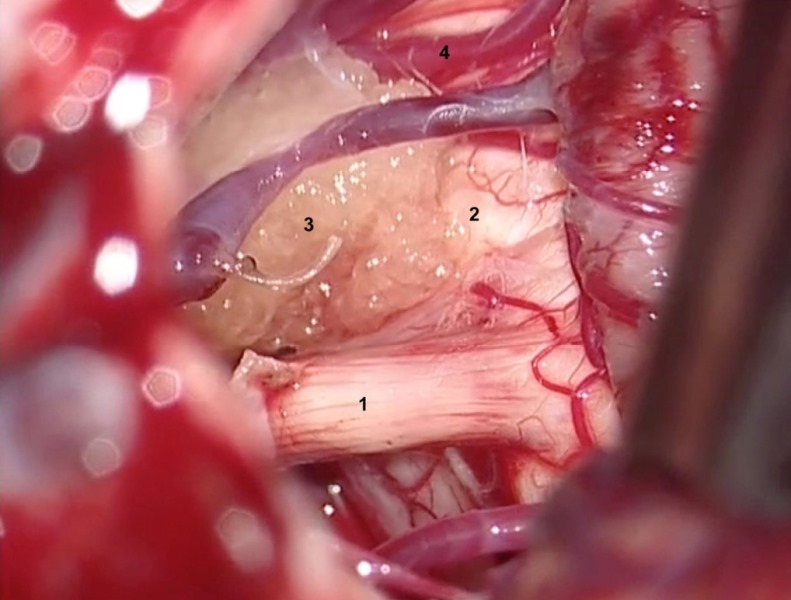
1. Trigeminal nerve.
2. Pons.
3. Superior cerebellar artery compressing the nerve medially.
4. Superior petrous vein.
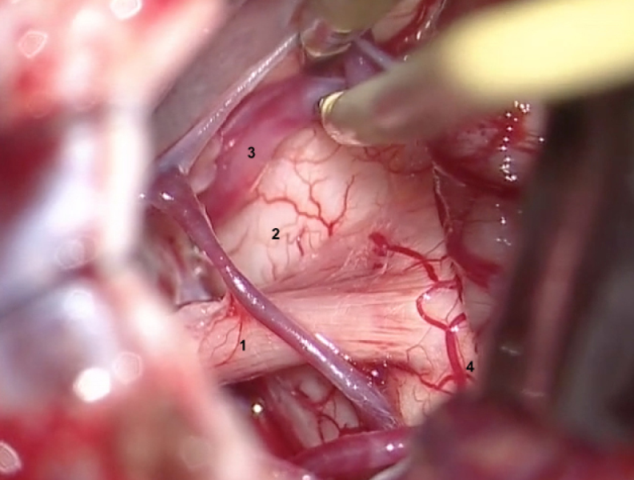
1. Trigeminal nerve.
2. Pons.
3. Superior cerebellar artery.
4. Middle cerebellar peduncle.
4. The artery most frequently compressing the trigeminal nerve is the superior cerebellar artery.
5. It is imperative to check the nerve at the junction with the brainstem for any residual compression before the next step.
6. The insulating material is interposed between the nerve and the vessel to prevent compression. In our service we use Teflon fragments (Figure 6 D).
7. If the procedure is for failed microvascular decompression and partial nerve division is desired, the nerve is somatotopically organised with V1 fibres superiorly and V3 inferiorly. If the goal is total removal of the pain pathways and there is concern about pain conduction through auxiliary pathways, consider also dividing the motor root.
Closure.
Anatomical reconstruction by planes is performed:
1. Apply bone wax to the edges of the craniectomy to prevent cerebrospinal fluid fistulae, mainly from mastoid cells20.
2. Perform airtight closure of the dura mater with continuous suture with 4-0 nylon.
3. The bony defect should be covered to reduce the possibility of pain associated with craniectomy.
4. The muscle is sutured with continuous stitches (Vicryl 3-0).
5. The skin is sutured with simple continuous stitches (Nylon 4-0).
Discussion
Microvascular decompression offers acceptable results, such that in 75% to 80% of patients the result can be described as successful; in a further 10% the result is good although total relief is not achieved. Therefore, its initial failure rate is around 10%. The success of this procedure is related to the previous duration of the clinic: when the evolution of symptoms is longer than eight years, the proportion of patients who obtain initial relief decreases significantly. Thus, the longer one waits to perform microvascular decompression, the lower the success rate. For some authors, this time should not exceed two years. On the other hand, it should be noted that it is less effective in patients with all three trigeminal branches affected or in those who have previously undergone a rhizotomy procedure 21,22. It is expected that 80% of patients will be pain free or have minor recurrence at 5 years and 70% at 8.5 years (therefore, the recurrence rate is approximately 5% each year); those patients in whom venous compression of the nerve is observed in the surgical field have more recurrences than those in whom arterial compression was observed.
Microvascular decompression should not cause post-surgical sensory deficit, although root manipulation may cause some dysesthesia in a low proportion of patients who rarely or never reach the degree of painful anesthesia. Thus, the incidence of facial anesthesia is much lower than with percutaneous procedures23,20,24.
Since it is a posterior fossa surgery, mortality is between 0.22% and 2%.
In terms of morbidity, the following should be noted: Aseptic meningitis occurs in 20% of cases, usually debuting three to seven days after the procedure, and clinically manifesting with headache, meningismus, pleocytosis and negative CSF culture, responding to steroids; major neurological morbidity appears in 1% to 10% of cases, including: deafness (1%), vestibular nerve dysfunction and facial nerve dysfunction; cranial nerve palsies: IV cranial nerve palsies producing diplopia (in 4.3%, mostly transient), VIII (in 3% of cases, causing hearing loss) and VII (in 1.6%, also mostly transient); postoperative haemorrhages, including subdural haematoma, intracerebral haematoma and subarachnoid haemorrhage; seizures and status epilepticus and cerebral infarction in the territory of the posterior cerebral artery or in the brain stem.
The general indications for microvascular decompression can be summarised under three headings1:
Patients under 70 years of age with trigeminal neuralgia, with an estimated survival of 5 years or more, with no significant medical or surgical risk factors.
Can be used in patients who have intractable pain despite previous percutaneous procedures.
V1 neuralgia, in patients for whom the risk of keratitis from exposure due to corneal anaesthesia is unacceptable (e.g. patients with blindness in the contralateral eye) or in those subjects who wish to avoid facial anaesthesia for any reason.
Stereotactic radiosurgery can also be used as a therapeutic option, recommended for patients with comorbidities, high-risk medical conditions, pain refractory to previous surgery, or those with anticoagulation25.
Management of therapeutic failures
90% of recurrences occur in the same distribution of previously affected branches; in the remaining 10%, these recurrences appear in a new trigeminal division and may represent a progression of the underlying process.
Percutaneous procedures can be repeated in patients who suffer a recurrence and who preserve some facial sensation. Repetition of such a percutaneous procedure is often effective.
When percutaneous techniques definitely fail, microvascular decompression can be performed but the success rate of microvascular decompression may be reduced, being about 90% in patients undergoing such a procedure for the first time and 43% for those undergoing microvascular decompression after a percutaneous technique (it should be noted, however, that this 90% rate may be overestimated and it should also be borne in mind that the group of patients in whom percutaneous procedures failed may select a subgroup of patients whose neuralgia is more difficult to treat)26.
Microvascular decompression can also be repeated in patients who suffer a recurrence after a first microdecompression, taking into account that the material interposed between the vascular structure and the nerve may have ceased to perform its function due to slippage or for any other reason27.
References
1. Greenberg Mark S. Handbook of Neurosurgery. Neuropathic Pain Sindromes- Trigeminal Neuralgia Six edition. Lake Land Florida. 2006; pp 378-386.
2. Van Loveren, H., Tew, J.M. Jr, Keller, J.T., Nurre, M.A.: A 10-year experience in the treatment of trigeminal neuralgia. Comparison of percutaneous stereotaxic rhizotomy and posterior fossa exploration. J Neurosurg 1982; 57: 757-764.
3. Katusic, S., Beard, C.M., Bergstralh, E., Kurland, L.T.: Incidence and clinical features of trigeminal neuralgia, Roche- ster, Minnesota, 1945-1984. Ann Neurol 1990; 27: 89-95.
4. Morley, T.P.: General considerations, medical therapy and minor operative procedures for trigeminal neuralgia. En Youmans JR (ed). Neurological Surgery, 3rd ed. Philadelphia; WB Saunders, 1990; pp. 3880-3887.
5. Morley, T.P.: General considerations, medical therapy and minor operative procedures for trigeminal neuralgia. En Youmans JR (ed). Neurological Surgery, 3rd ed. Philadelphia; WB Saunders, 1990; pp. 3880-3887.
6. Bullitt, E., Tew, J.M., Boyd, J.: Intracranial tumors in patients with facial pain. J Neurosurg 1986; 64: 865-871.
7. Young, R.F.: The trigeminal nerve and its central pathways. Physiology of facial sensation and pain. En Rovit RL, Murali R, Jannetta PJ (eds). Trigeminal neuralgia. Balti- more; Williams and Wilkins, 1990; pp. 27-51.
8. Humphrey, T. The central relations of trigeminal nerve. En Kahn EA, Crosby EC, Schneider RC, Taren JA (eds). Correlative neurosurgery, 20th ed. Springfield; Charles C Tomas, 1973; pp. 477-492.
9. Hardy DG, Rhoton AL. Microsurgical Relationships of the Superior Cerebellar Artery and the Trigeminal Nerve. J Neurosurg. 1978; 49:669–678
10. Morita A, Fukushima T, Miyazaki S, et al. Tic Douloureux Caused by Primitive Trigeminal Artery or its Variant. J Neurosurg. 1989; 70:415–419
11. Apfelbaum RI, Carter LP, Spetzler RF, Hamilton MG. In : Trigeminal Neuralgia: Vascular Decompression. Neurovascular Surgery. New York: McGraw-Hill; 1995:1107–1117
12. Sweet, W.H.: The treatment of trigeminal neuralgia (tic douloureux). N Engl J Med 1986; 315: 174-177.
13. Sweet, W.H.: The history of the development of treatment for trigeminal neuralgia. Clin Neurosurg 1985; 32: 294- 318.
14. Hakanson, S.: Trigeminal neuralgia treated by the injection of glycerol into the trigeminal cistern. Neurosurgery 1981; 9: 638-646.
15. Shelden, C.H., Pudenz, R.H., Freshwater, D.B., Crue, B.L.: Compression rather than decompression for trigeminal neuralgia. J Neurosurg 1955; 12: 123-126.
16. Mullan, S., Lichtor, T.: Percutaneous microcompres- sion of the trigeminal ganglion for trigeminal neuralgia. J Neurosurg 1983; 59: 1007-1012.
17. Jannetta, P.J.: Arterial compression of the trigeminal nerve at the pons in patients with trigeminal neuralgia. J Neurosurg 1967; 26: S159-162.
18. McLaughlin MR, Jannetta PJ, Clyde BL, Subach BR, Comey CH, Resnick DK. Microvascular decompression of cranial nerves: Lessons learned after 4400 operations. J Neurosurg. 1999; 90:1–8
19. Tew JM, van Loveren HR. Atlas of Operative Microneurosurgery. Philadelphia: W. B. Saunders; 1994; 1: Aneurysms and Arteriovenous Malformations
20. Jannetta, P.J.: Microsurgical management of trigeminal neuralgia. Arch Neurol 1985; 42: 800.
21. Alberione, F., Arena, A., Matera, R.: [Microvascular descompression for trigeminal neuralgia: prognostic [correc- ted] factors]. Neurocirugia 2008; 19: 242-247.
22. Burchiel, K.J., Clarke, H., Haglund, M., Loeser, J.D.: Longterm efficacy of microvascular decompression in trigeminal neuralgia. J Neurosurg 1988; 69: 35-38.
23. Hanakita, J., Kondo, A.: Serious complications of microvascular decompression operations for trigeminal neu- ralgia and hemifacial spasm. Neurosurgery 1988; 22: 348- 352.
24. Schmidek, H.H., Sweet, W.H. (eds): Operative neurosurgical techniques, 20th ed. Philadelphia; WB Saunders, 1988
25. Lunsford LD. Comment on Taha J M and Tew J M:Comparison of Surgical Treatmen ts for Trigeminal Neuralgia: Reevaluation of Radiofrequency Rhizotomy. Neurosurgery. 1996; 38
26. Barba, D., Alksne, J.F.: Success of microvascular decompression with and without prior surgical therapy for trigeminal neuralgia. J Neurosurg 1984; 60: 104-107.
27. Ferna4ndez-Carballal, C., Garci4a-Salazar, F., Perez- Calvo, J., Garcia-Leal, R., Gutierrez, F.A., Carrillo, R.: [Management of recurrent trigeminal neuralgia after failed microvascular decompression]. Neurocirugia 2004; 15: 345- 352.
Alternative link

