Intranasal dexmedetomidine in obstetrics and gynecology sedation
Dexmedetomidina intranasal en sedación para ginecoobstetricia
Gaceta Médica Boliviana, vol. 43, no. 1, 2020
Universidad Mayor de San Simón
Articulos Originales
Received: 22 February 2020
Accepted: 20 May 2020

Abstract:
Objectives: to evaluate the use of intranasal dexmedetomidine as an adjuvant for sedation in LUI and MVA associated with a target-controlled infusion system.
Methods: uncontrolled, prospective, single-blind, prospective clinical trial. Sample of 48 patients meeting inclusion criteria. CD group was administered Dexmedetomidine IN at a dose of 0.9 µg/kg and another control group. For statistical analysis of continuous variables, mean and SD were used; for ordinal variables, frequency was calculated. Student’s t-test and chi χ2 were used. Confidence level 95% and margin of error 12%.
Results: mean age 32 ± 7 years; in the CD group the induction and maintenance dose of remifentanil was 2 ± 0.7 ng/ml and in the SD group the induction dose was 4.1 ± 0.7 ng/ml and maintenance 3.9 ± 0.5 ng/ml; for propofol the induction and maintenance dose was 3.7 ± 0.5 mcg/ml in the SD group while in the CD group the induction and maintenance dose was 2.1 ± 0.5 mcg/ml. Heart rate was 64-62 and in the SD group it was 70-67 beats/min. The MAP associated with dexme is 73 mmHg and the SD group is 78 mmHg. There were no complications due to the administration of dexmedetomidine.
Conclusions: IN Dexmedetomidine allows to decrease the dose of drugs, with stability of haemodynamic changes, the dose used does not produce complications.
Keywords: dexmedetomidine, intranasal administration, deep sedation, curettage.
Resumen:
Objetivos: evaluar el uso de la dexmedetomidina intranasal como coadyuvante para sedación en LUI y AMEU asociado al sistema de infusión controlado por objetivo.
Métodos: ensayo clínico no controlado, prospectivo y simple ciego. Muestra de 48 pacientes que cumplen los criterios de inclusión. Grupo CD se administró Dexmedetomidina IN a dosis de 0,9 µg/kg y otro grupo de control. Para el análisis estadístico de variables continuas se usó media y DE; para variables ordinales se calculó frecuencia. Además de prueba T de Student y Chi χ2. Nivel de confianza de 95 % y margen de error 12%.
Resultados: edad media de 32 ± 7 años; en el grupo CD la dosis de inducción y mantenimiento de remifentanil fue de 2 ± 0,7 ng/ml y en el grupo SD la dosis de inducción fue de 4,1 ± 0,7 ng/ml y de mantenimiento 3,9 ± 0,5 ng/ml; para el propofol la dosis de inducción y mantenimiento fue 3,7 ± 0,5 mcg/ml en el grupo SD mientras que, en el grupo CD la dosis de inducción y mantenimiento fue de 2,1 ± 0,5 mcg/ml. La frecuencia cardiaca de 64 – 62 y en el grupo SD fue de 70 – 67 latidos/min. La PAM asociado al dexme está por 73 mmHg y el grupo de SD es de 78 mmHg. No se verifico complicaciones por la administración de la Dexmedetomidina.
Conclusiones: la dexmedetomidina IN permite disminuir la dosis de los fármacos, con estabilidad de los cambios hemodinámicos, la dosis utilizada no produce complicaciones.
Palabras clave: dexmedetomidina, administración intranasal, sedación profunda, legrado.
Dilatation and curettage is a common procedure in obstetrics and gynaecology, with short hospital stay and discharge1.The most common methods to reduce pain during cervical dilatation and uterine interventions are paracervical block and sedation2.
The culmination of first trimester abortion is suction evacuation, a minor surgical procedure in gynaecology and obstetrics associated with preoperative pain and anxiety3. The choice of technique and analgesia depends on the effectiveness, cost, safety and side effects for the patient4. Although there are other anaesthetic techniques such as general anaesthesia, regional anaesthesia and local anaesthesia5. The titration of anaesthetic doses should be performed with care and patients should be continuously monitored6. Dexmedetomidine is an α2-adrenergic agonist that provides conscious sedation, analgesia, anxiolytic and adjuvant anaesthesia7, Common methods of administration of dexmedetomidine are intravenous, intranasal and intramuscular. Intranasal dexmedetomidine is fast-acting, easy to use, odourless, painless and does not require an intravenous route8. Nasal administration avoids the first-pass effect9. The absolute bioavailability of dexmedetomidine is 65% (35 - 93%) after intranasal administration in adults10. The aim of the present study is to evaluate the use of intranasal dexmedetomidine as an adjuvant for sedation in sharp curettage and manual vacuum aspiration associated with the Target Controlled Infusion (TCI) system.
Material and methods
This is a non-controlled, longitudinal, prospective, single-blind, clinical trial at the Hospital Obrero N° 2 “Caja Nacional de Salud”, using a protocol of care approved by the obstetric anaesthesiology service belonging to the Gyneco-obstetrics department, during the period from November 2019 to March 2020. A total population of 139 patients was determined for the study. The sample consisted of 48 patients undergoing Instrumental Uterine Curettage (LUI) and Manual Endo Uterine Aspiration (MVA), who met the following inclusion criteria: age between 18 and 45 years, in addition to fasting for solid food for more than 6 hours and liquids for more than 2 hours, haemodynamic stability, an ASA (American Society of Anesthesiologists) type I and II physical status classification, a weight of less than 80 kilograms and who agreed to participate in the study. Patients who did not comply with fasting hours, patients with mental alteration, heart disease, presence of nasal mass, excessive use of sedatives, allergy to the drugs to be administered, weight greater than 80 kilograms, haemodynamic instability, ASA physical status classification greater than III, or who refused to participate in the study were excluded from the study.
They were divided into two groups:
Dexmedetomidine group (DC): 24 patients, administered intranasally at a dose of 0.9 µg/kg. (45 minutes before the procedure).
Group without Dexmedetomidine (SD): 24 patients, control group.
All patients underwent pre-anaesthetic assessment and informed consent to participate in the study.
The patients were admitted to the curettage room after placement of an 18 gauge (Gauge) intravenous line at 10 ml/kg/hr, connected to two three-way valves and previously (45min) premedicated with intranasal dexmedetomidine at a dose of 0.9 µg/kg with the aid of an insulin syringe for atomisation. It was recommended not to wipe the nose immediately or sneeze after administration. Prior to admission to the curettage room, monitoring and surveillance of heart rate and oxygen saturation by pulse oximetry is performed from the time of premedication. Throughout the procedure, blood pressure, heart rate, pulse oximetry and oxygen administration at 4 litres per minute with a face mask are monitored. For preventive analgesia, Ketorolac 0.5 mg/kg intravenous was administered as a single dose.
For the anaesthetic protocol:
B Braun Perfusor® Space infusion pumps were used for anaesthetic induction and maintenance, with input of each patient’s anthropometric data and the pharmacokinetic and pharmacodynamic parameters provided by the Target Controlled Infusion (TCI) system. The Minto model is selected for remifentanil between 2 - 3 ng/ml over three minutes (effect site concentration) and the Schnider model between 2-3 mcg/ml. (effect site concentration) for propofol.
During the maintenance period ventilation support was provided if the patient remained apnoeic.
Drug complications were defined as:
Hypotension is reduction of mean arterial pressure by more than 20%, treated with vasopressor.
Bradycardia is a reduction of 50 beats per minute and its treatment with atropine 0.01 µg/kg.
Oxygen desaturation below 90% for 10 seconds and is supported with supplemental oxygen.
Respiratory depression is caused by both a decrease in respiratory rate and tidal volume.
Apnea was defined as the absence of spontaneous breathing for more than 30 seconds.
In recovery rooms, the degree of sedation is assessed using the Ramsay scale, pain is assessed using the Visual Numerical Scale (EVN) and haemodynamic monitoring is continued using pulse oximetry, heart rate and blood pressure.
Statistical analysis
The statistical analysis for continuous variables such as age, Body Mass Index (BMI), weight, height, heart rate, Systolic Blood Pressure, Diastolic Blood Pressure, Mean Arterial Pressure (MAP), oxygen saturation, induction and maintenance doses of both drugs, mean and standard deviation were performed
In the case of ordinal variables such as the Ramsay scale, American Society of Anaesthesiologists (ASA) physical status classification and the Visual Numerical Scale (VNS) measurement; nominal variables such as complications were calculated as frequencies or percentages. Student’s t-test was applied in the comparison of continuous variables according to groups; for nominal variables, the Chi-Square test (χ2) was applied. With a confidence level of 95% and a margin of error of 12%.
All statistical analysis was recorded and analysed in SSPS version 25 for Windows and data tabulation in Microsoft Excel 2016.
Ethical considerations
The ethical considerations of the study are based on the Declaration of Helsinki, updated in 2013. Therefore, the personal data of the patients and the obtaining of informed consent are kept confidential.
Results
The results of both study groups were analysed in terms of demographic characteristics, mean age in both groups was 32 ± 7 years, not significan
BMI in the no dexme group was 28 ± 6 kg/cm2 while in the dexme group it was 27 ± 3 kg/cm2 , not significant.
The induction dose of remifentanil in the no dexme group was 4.1 ± 0.7 ng/ml and in the dexme group it was 2 ± 0.7 ng/ml. With a dose reduction of 50%. Similarly, during maintenance it was 3.9 ± 0.5 ng/ml in the group without dexme and for the group with dexme it was 2 ± 0.7 ng/ml, with a significance of 0.000. The reduction was 48.7%. On the other hand, the induction dose of propofol in the no dexme group was 3.7 ± 0.6 mcg/ml and in the dexme group it was 2.1 ± 0.5 mcg/ml. And the maintenance dose in the no dexme group was 3.7 ± 0.5 mcg/ml and for the dexme group it was 2.1 ± 0.8 mcg/ml. Likewise, the maintenance dose was reduced by 43.2% in the dexme group, being significant in both times of 0.000.
Surgical time was almost similar in both groups: 12.4 ± 3.4 min (no dexme group) and 11.9 ± 1.9 min (dexme group). Similarly, the anaesthetic time was 14 min in both groups and the recovery room discharge time was 11 min. These three evaluation times were not significant (Table 1).
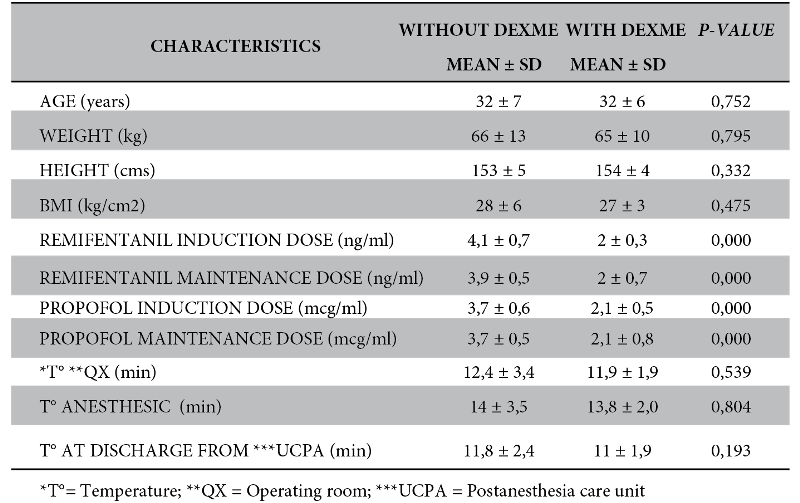
To continue, the basal heart rate in the no dexme group was 74 ± 7.8 beats/min and in the dexme group it was 71 ± 9.2 beats/min, not significant. After administration of dexmedetomidine, a decrease of 11% was observed compared to the no dexme group; at the time of induction and maintenance, lower heart rates were observed, but not bradycardia (64 to 62 beats/min) in the dexme group, while the no dexme group maintained a heart rate between 70 to 67 beats/min. This is significant (Figure 1).
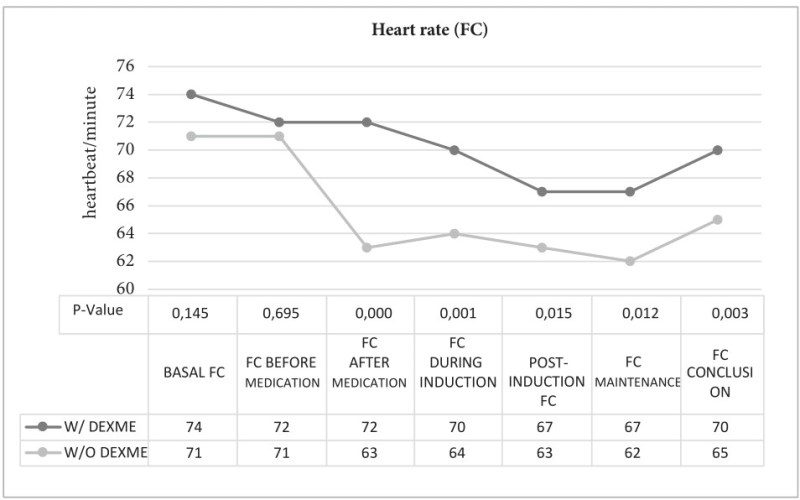
With respect to baseline mean arterial blood pressure, it was similar in both groups 81-82 mmHg, where significance was observed during induction (no dexme group: 79 mmHg and dexme group: 74 mmHg) and after induction (no dexme group: 78 mmHg and dexme group: 73 mmHg), p-value 0.017 and 0.016 respectively (Figure 2).
Oxygen saturation was maintained between 96% and 97% with supplemental oxygen support at 4 litres/minute.
The Ramsay sedation scale at drug administration was found with a predominance of Ramsay 2 in 17 patients; in the recovery room in both groups 17 patients were found with Ramsay 2.
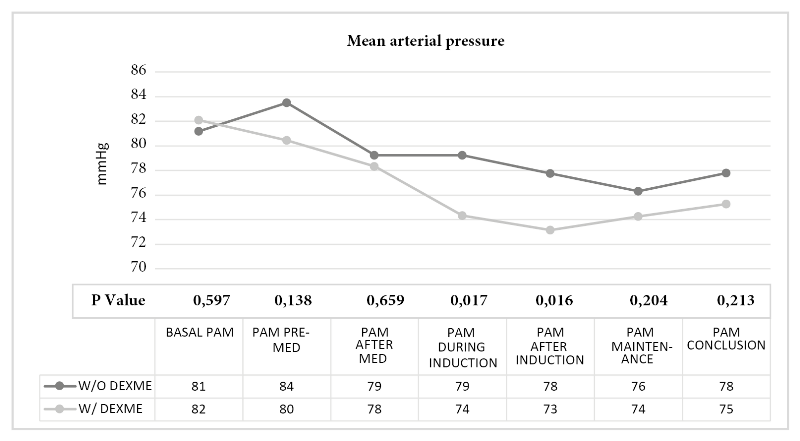
In relation to the Visual Numerical Scale (EVN) in the group without dexme 21 patients were found with EVN of 0 and in the group with dexme 18 patients were found, only one patient was reported with an EVN of 3. With a Chi χ2 of 0.357 (Figure 3).
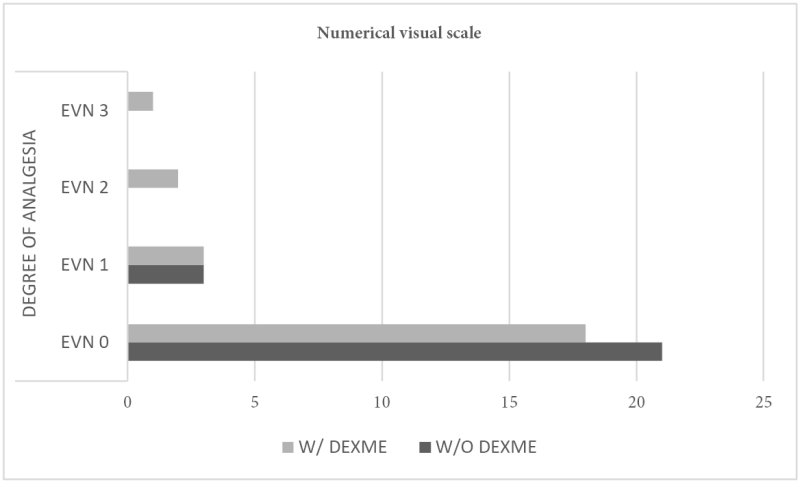
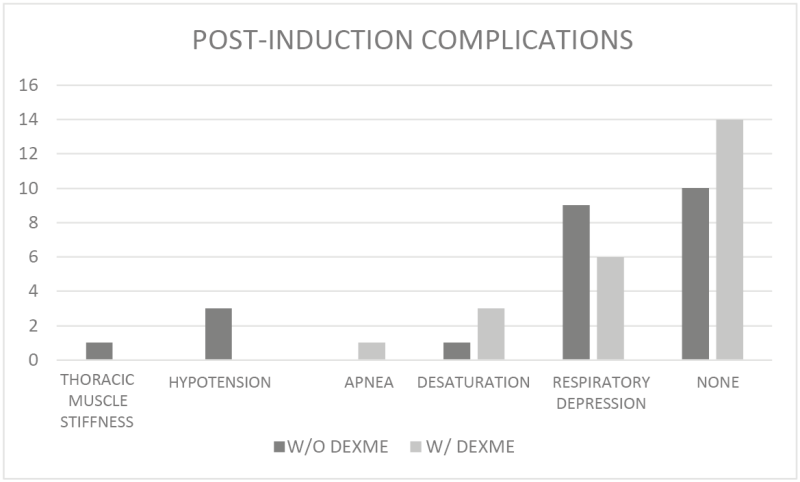
Finally, there were no complications posed by the administration of dexmedetomidine, but it was observed that after induction with remifentanil and propofol, 1 patient with woody chest and 3 patients with arterial hypotension in the group without dexme; 1 patient with apnoea in the group with dexme; 9 patients presented respiratory depression in the group without dexme and 6 patients in the group with dexme; 14 patients did not present complications in the group with dexme and 10 patients in the group without dexme. Chi χ2 of 0.202 (Figure 4).
Discussion
The present study evaluates the addition of intranasal dexmedetomidine as an adjuvant for sedation in instrumental curettage and manual uterine aspiration associated with the target-controlled infusion system. Regarding the mean age of both groups is 32 ± 7 years, Canache et al11 reported a mean age of 29 ± 8 years in the dexmedetomidine group and 25 ± 6 years in the propofol group.
Based on the induction dose of remifentanil and propofol was reduced by 50 % and 43.2 % respectively in the dexmedetomidine group, which reduces the site concentration effect of the induction drugs, Wan et al12 mention in their study the reduction of the concentration requirements of remifentanil and propofol during induction. Similarly Shi et al3 allude to a reduction in propofol consumption in the intranasal dexmedetomidine group at 1 µg/kg and thus complete a sparing effect of propofol use.
For Hernandez et al13 the use of Dex infusion as an adjuvant reduces the consumption of anaesthetic drugs. In the study, the use of dexmedetomidine at even lower doses such as 0.9 µg/kg helps to reduce the use of drugs not only in the induction phase but also in the maintenance phase. The surgical time is 12 min (no dexme group) and 11 min (dexme group), due to the fact that the hospital is a medical residency training centre. Islam et al14 reported 6.5 minutes for AMEU procedures.
The recovery room discharge time is 11 minutes in both groups and Fouad18et al15 postulate a recovery time of 11.9 min in the dexmedetomidine-fentanyl group. While for Tripathi et al16 the recovery time is shorter at 5.63 minutes in the dexmedetomidine group. As proposed by Virendra et al17 allows for rapid recovery and early ambulation with dexmedetomidine administration.
The addition of intranasal dexmedetomidine produces haemodynamic stability with a heart rate of at least 62 beats/minute, similarly Sattari et al18 state haemodynamic stability, while El Sharkawy19 argues bradycardia in the dexmedetomidine-ketamine group versus the other propofolketamine group, attributed to reduced noradrenaline release.
With regard to mean arterial pressure, the study shows a decrease in PAM (mean blood pressure) associated with the use of dexmedetomidine, which is considered significant and that associated with remifentanil and propofol can lead to a decrease in PAM at different times of evaluation, due to the Schnider model. For Fouad et al15 expresses a better preservation of PAM and Oxygen Saturation in the dexmedetomidine group at 1 µg/kg.
In reference to the Ramsay Sedation Scale, Ramsay 2 was preponderant in the recovery room, while Garcia et al20 reveal that it produced sedation with the administration of intranasal dexmedetomidine 1 µg/kg with respect to the baseline value of the Ramsay scale obtained.
With respect to the Visual Numerical Scale, a decrease in pain was observed in the group without dexme, while Shi et al3 reported effective analgesia, Sattari et al18 reported more pain in the control group (placebo), it should be noted that the author used intranasal dexmedetomidine 1 µg/kg. It is deduced from the administered dose of 0.9 µg/kg that it does not produce adequate analgesia.
For Hernandez et al13the use of Dex infusion as an adjuvant reduces the consumption of anaesthetic drugs. In the study, the use of dexmedetomidine at even lower doses such as 0.9 µg/kg helps to reduce the use of drugs not only in the induction phase but also in the maintenance phase. The surgical time is 12 min (no dexme group) and 11 min (dexme group), due to the fact that the hospital is a medical residency training centre. Islam et al14 reported 6.5 minutes for AMEU procedures.
The recovery room discharge time is 11 minutes in both groups and Fouad18et al15 postulate a recovery time of 11.9 min in the dexmedetomidine-fentanyl group. While for Tripathi et al16 the recovery time is shorter at 5.63 minutes in the dexmedetomidine group. As proposed by Virendra et al17 allows for rapid recovery and early ambulation with dexmedetomidine administration.
The addition of intranasal dexmedetomidine produces haemodynamic stability with a heart rate of at least 62 beats/minute, similarly Sattari et al18 state haemodynamic stability, while El Sharkawy19 argues bradycardia in the dexmedetomidine-ketamine group versus the other propofolketamine group, attributed to reduced noradrenaline release.
With regard to mean arterial pressure, the study shows a decrease in PAM (mean blood pressure) associated with the use of dexmedetomidine, which is considered significant and that associated with remifentanil and propofol can lead to a decrease in PAM at different times of evaluation, due to the Schnider model. For Fouad et al15 expresses a better preservation of PAM and Oxygen Saturation in the dexmedetomidine group at 1 µg/kg.
In reference to the Ramsay Sedation Scale, Ramsay 2 was preponderant in the recovery room, while Garcia et al20 reveal that it produced sedation with the administration of intranasal dexmedetomidine 1 µg/kg with respect to the baseline value of the Ramsay scale obtained.
With respect to the Visual Numerical Scale, a decrease in pain was observed in the group without dexme, while Shi et al3 reported effective analgesia, Sattari et al18 reported more pain in the control group (placebo), it should be noted that the author used intranasal dexmedetomidine 1 µg/kg. It is deduced from the administered dose of 0.9 µg/kg that it does not produce adequate analgesia.
No complications were reported with the administration of dexmedetomidine. Similarly, Sethi et al21 state that there were no intraoperative or postoperative adverse effects in the dexmedetomidine group. However, Garcia et al20 clarify that dexmedetomidine is not a ventilatory depressant and therefore no alteration in the respiratory pattern occurred.
No complications were reported with the administration of dexmedetomidine. Similarly, Sethi et al21 state that there were no intraoperative or postoperative adverse effects in the dexmedetomidine group. However, Garcia et al20 clarify that dexmedetomidine is not a ventilatory depressant and therefore no alteration in the respiratory pattern occurred.
Regarding a case of apnea in the dexme group, Wanderer et al22 emphasise that mild to moderate sedation with dexmedetomidine does not offer protection against central apnoea and airway obstruction. Meanwhile, Alizadehasl et al23 note no respiratory depression in the IV dexmedetomidine group similar to the report in the study by Fouad et al15. Tomar et al report that respiratory depression is not due to dexmedetomidine because its effects are not mediated by γ-amino butyric acid.
In conclusion, the use of an adjuvant such as intranasal dexmedetomidine allows for a reduction of anaesthetics during the induction and maintenance stage associated with the TCI infusion system, which allows for a more precise administration. The haemodynamic changes produced by dexmedetomidine do not lead to severe side effects, which may be due to the fact that nasal absorption is more gradual and the dose used, but it should be noted that it does not produce adequate analgesia.
The use of intranasal dexmedetomidine as premedication is recommended even without the availability of venous access, since at the dose mentioned there are no complications.
References
1. Tas A, Mistanoglu V, Darcin, S, Kececioglu M. Tramadol versus fentanyl during propofol-based deep sedation for uterine dilatation and curettage: A prospective study; Journal of Obstetrics and Gynaecology Research, [Internet]. 2014 [Citado el 14 de abril de 2020]; 40(3), 749–753. Disponible en: https://doi.org/10.1111/jog.12259
2. Asgari Z, Razavi M, Hosseini R, Nataj M, Rezaeinejad M, Sepidarkish M. Evaluation of Paracervical Block and IV Sedation for Pain Management during Hysteroscopic Polypectomy: A Randomized Clinical Trial. Pain Research and Management [Internet]. 2017[Citado el 14 de abril de 2020]: 1–7. Available from: https://doi.org/10.1155/2017/5309408
3. Shi H, Yang D, Liu J. Intranasal dexmedetomidine in termination of first trimester pregnancy of suction evacuation; Asian Journal of Anesthesiology [Internet]. 2017[Citado el 03 de noviembre de 2019]:1–7. Available from: https://doi.org/10.1016/j.aja.2017.09.001
4. Calvache JA, Delgado MF, Giraldo A, Salamanca N. Anaesthesia for evacuation of incomplete miscarriage; Cochrane Database of Systematic Reviews [Internet]. 2010 [Citado el 8 de abril de 2020]: 9. Art. No.: CD008681. Available from https://doi.org/10.1002/14651858.CD008681.pub2
5. Mittal P, Goyal M. Pain relief during minor procedures: a challenge for gynaecologists; Indian Journal Med Res [Internet]. 2015[Citado el 14 de abril de 2020]: October, 366–68. Available from: https://doi.org/10.4103/0971-5916.169192
6. Da´meh S, Adaileh M, Almajali A, El-Share K, Shhadat K. USAGE OF VARIOUS CONCENTRATIONS OF KETAFOL FOR KETAFOL FOR DILATATION AND CURETTAGE; Indian Journal of Medical Research and Pharmaceutical Sciences [Internet]. 2016 [Citado el 14 de abril de 2020]: 3; 33–38. Available from: https://doi.org/10.5281/zenodo.163586
7. Asmussen Sven, Maybauer DM, Frase JF, Jennings K, George S, Maybauer MO. A meta-analysis of analgesic and sedative effects of dexmedetomidine in burn patients; J Burn [Internet]. 2013 [citado el 10 de abril de 2020]; 39 (4):625-631. Available from: https://doi.org/10.1016/j.burns.2013.01.008
8. Jayaraman L, Sinha A, Punhani D. A comparative study to evaluate the effect of intranasal dexmedetomidine versus oral alprazolam as a premedication agent in morbidly obese patients undergoing bariatric surgery: J Anaesthesiol Clin Pharmacol [Internet]. 2013 [Citado el 14 de abril de 2020]; 29(2):179-182. Available from: https://doi.org/10.4103/0970-9185.111680
9. Li LQ, Wang C, Xu HY, Lu HL, Zhang HZ. Effects of different doses of intranasal dexmedetomidine on preoperative sedation and postoperative agitation in pediatric with total intravenous anesthesia undergoing adenoidectomy with or without tonsillectomy. Medicine (Baltimore). [Internet]. 2018 [citado el 5 de diciembre de 2019];97(39): e12140. Available from: https://doi.10.1097/MD.0000000000012140
10. Iirola T, Vilo S, Manner T, Olkkola KT. Bioavailability of dexmedetomidine after intranasal administration; Eur J Clin Pharmacol [Internet]. 2011 [Citado el 14 de abril de 2020]; 67: 825–831. Available from: https://doi.org/10.1007/s00228-011-1002-y
11. Canache Melgarejo OC, De Andrade Goncalves C. Legrados Uterinos: cambios hemodinámicos en pacientes bajo sedación con dexmedetomidina y propofol. Caracas, Venezuela [Imternet]. 2014 [Citado el 31 de octubre de 2019]; 1-52. Available from: http://saber.ucv.ve/handle/10872/18409
12. Wan Hassan WMN, Tan HS, Mohamed Zaini RH. Comparison of the effects of dexmedetomidineon the induction of anaesthesia using Marsh and Schnider pharmacokinetic models of propofol target-controlledinfusion; Malays J Med Sci [Intenert]. 2018 [Citado el 01 de noviembre de 2019];25(1):24–31. Available from: https://doi.org/10.21315/mjms2018.25.1.4
13. Hernández-Bernal CE. Anestesia con infusión de dexmedetomidina en cirugía maxilofacial; Rev Mex Anest [Internet].2016 [Citado el 15 de abril de 2020]; 39 (suppl:1): 117–120. Available from: https://www.medigraphic.com/cgi-bin/new/resumen.cgi?IDARTICULO=66178
14. Islam R, Biswas SP, Halder D, Fatima K. Safety & efficacy of manual vacuum aspiration compared to dilatation & curettage in the management of early pregnancy failure; Bang Med J Khulna [Internet]. 2017 [citado el 10 de abril de 2020]; 49 (1-2):18–22. Available from: https://doi.org/10.3329/bmjk.v49i1-2.31820
15. Fouad G, Ali R. M, Elsayed A, Ismail A, Eshak B, Hanna A. Comparative evaluation of hemodynamic stability and recovery during conscious sedation by dexmedetomidine with fentanyl versus ketamine with fentanyl in dilatation and curettage. The Egyptian Journal of Hospital Medicine [Internet]. 2018 [citado el 10 de octubre de 2020]; 73 (2):5992- 5997. Available from: https://doi.org/10.12816/ejhm.2018.12367
16. Tripathi M. Kumar V, Kalashetty MB, Malviya D, Bais P S, Sanjeev OP. Comparison of Dexmedetomidine and Midazolam for Sedation in Mechanically Ventilated Patients Guided by Bispectral Index and Sedation-Agitation Scale; Anesthesia, essays and researches [Internet].2017 [Citado el 12 de abril de 2020]; 11(4), 828–833. Available from: https://doi.org/10.4103/aer.AER_48_17
17. Virendra S, Barya S, Chandra P, Goyal S, Kumar V, Petkar J. Comparative evaluation of hemodynamic stability and recovery during conscious sedation by dexmedetomidine with fentanyl v/s ketamine with fentanyl in dilatation and curettage; European Journal of Pharmaceutical and Medical Research [Internet]. 2017 [Citado el 10 de abril de 2020];4(6):719–724. Available from: https://www.ejpmr.com/home/abstract_id/2543
18. Sattari H, Hashemian M, Noroozi M, Khaloueipour. Evaluation of the effect of dexmedetomidine on hemodynamic changes and recovery time in patients undergoing dilatation and curettage. Prensa Med Argent [Internet].2019 [Citado el 25 de enero de 2020];105(5):1-3. Disponible en: https://www.scholarsliterature.com/journals/la-prensa-medica/fulltext/evaluation-of-the-effect-of-dexmedetomidine-on-hemodynamic-changes-and-recovery-time-in-patients-undergoing-dilatation-and-curettage
19. El Sharkawy RA. Efficacy of Adding Low Dose Ketamine to Dexmedetomidine Versus Low Dose Ketamine and Propofol for Conscious Sedation in Patients Undergoing Awake Fiber optic Intubation; Anesth Essays Res [Internet]. 2019 [Citado el 27 de octubre de 2019]; 13:73–8. Available from: https://doi.org/10.4103/aer.AER
20. Garcia C, Ramos J. Analgesia posoperatoria con dexmedetomidina intranasal en cirugía laparoscópica diagnóstica; Interciencia [Internet]. 2012 [Citado el 15 de abril de 2020]: 2(3)17–22. Available from: https://issuu.com/bruno_giol/docs/julio_2012
21. Sethi P, Sindhi S, Verma A, Tulsiani K. Dexmedetomidine versus propofol in dilatation and curettage: An open-label pilot randomized controlled trial; Saudi Journal of Anaesthesia [Internet]. 2015 [Citado el 31 de octubre de 2019];9(3), 258–262. Available from: https://doi.org/10.4103/1658-354X.154699
22. Wanderer J, Rathmell J. Comparing the Respiratory Effects of Dexmedetomidine and Propofol; Anesthesiology [Internet]. 2019 [Citado el 27 de octubre de 2019];131(5). Available from: https://doi.org/10.1097/ALN.0000000000003008
23. Alizadehasl A, Sadeghpour A, Totonchi Z, Azarfarin R, Rahimi S, Hendiani A. Comparison of sedation between dexmedetomidine and propofol during transesophageal echocardiography: A randomized controlled trial. Ann Card Anaesth [Internet].2019 [Citado el 27 de octubre de 2019];22:285-290. Available from: https://doi.org/10.4103/aca.ACA_42_18
Author notes
E-mail: wonderful122.paa@gmail.com
Conflict of interest declaration
Alternative link
http://www.scielo.org.bo/scielo.php?script=sci_arttext&pid=S1012-29662020000100006&lng=es&nrm=iso (html)

